The Role of Sirtuin1 in Regulating Endothelial Function, Arterial Remodeling and Vascular Aging
- PMID: 31572218
- PMCID: PMC6751260
- DOI: 10.3389/fphys.2019.01173
The Role of Sirtuin1 in Regulating Endothelial Function, Arterial Remodeling and Vascular Aging
Abstract
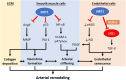
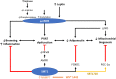
Sirtuin1 (SIRT1), which belongs to a highly conserved family of protein deacetylase, is one of the best-studied sirtuins. SIRT1 is involved in a variety of biological processes, including energy metabolism, cell proliferation and survival, chromatin dynamics, and DNA repair. In the vasculature, SIRT1 is ubiquitously expressed in endothelial cells, smooth muscle cells, and perivascular adipose tissues (PVAT). Endothelial SIRT1 plays a unique role in vasoprotection by regulating a large variety of proteins, including endothelial nitric oxide synthase (eNOS). In endothelial cells, SIRT1 and eNOS regulate each other synergistically through positive feedback mechanisms for the maintenance of endothelial function. Recent studies have shown that SIRT1 plays a vital role in modulating PVAT function, arterial remodeling, and vascular aging. In the present article, we summarize recent findings, review the molecular mechanisms and the potential of SIRT1 as a therapeutic target for the treatment of vascular diseases, and discuss future research directions.
Keywords: PVAT; SIRT1; eNOS; vascular aging; vascular remodeling.
Copyright © 2019 Man, Li and Xia.
Figures


References
Publication types
LinkOut - more resources
Full Text Sources

