Cerebrospinal Fluid CXCL10 as a Candidate Surrogate Marker for HTLV-1-Associated Myelopathy/Tropical Spastic Paraparesis
- PMID: 31572323
- PMCID: PMC6749079
- DOI: 10.3389/fmicb.2019.02110
Cerebrospinal Fluid CXCL10 as a Candidate Surrogate Marker for HTLV-1-Associated Myelopathy/Tropical Spastic Paraparesis
Abstract
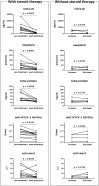
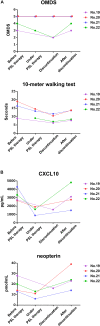
Human T-cell leukemia virus type 1 (HTLV-1)-associated myelopathy/tropical spastic paraparesis (HAM/TSP) is a debilitating, progressive disease without effective treatment; therefore, development of disease modifying therapy that improves long-term functional outcomes is an unmet need for patients. However, it is virtually impossible to consider this as a primary endpoint in clinical trials owing to the prolonged disease course. Therefore, development of surrogate markers that help predict the effectiveness of new interventions is essential. Currently, several candidate surrogate markers have been identified for HAM/TSP. Cerebrospinal fluid (CSF) C-X-C motif chemokine 10 (CXCL10) is involved in the pathogenesis of HAM/TSP and was shown to correlate with disease progression. However, it remains unclear whether changes in CSF CXCL10 levels are observed in response to treatment and whether these correlate with prognosis. Here we investigated several markers, including CSF CXCL10, in this respect. Data pertaining to patient characteristics and results of motor function evaluation and CSF examination of 13 HAM/TSP patients who received steroid treatment were retrospectively analyzed. Osame motor disability scores (OMDS), 10 m walking time, and CSF levels of CXCL10, neopterin, total protein, cell counts, and anti-HTLV-1 antibody titer were compared before and after steroid therapy. Levels of all CSF markers, with the exception of cell count, were significantly decreased after treatment. Nine of the 13 patients (69.2%) showed improvement in OMDS and were considered responders. Pre-treatment CSF levels of CXCL10 and anti-HTLV-1 antibody titer in responders were higher than those in non-responders (p = 0.020 and p = 0.045, respectively). Patients who continued low-dose oral prednisolone maintenance therapy after methylprednisolone pulse therapy showed sustained improvement in OMDS and CSF CXCL10 and neopterin levels lasting for 2 years. In contrast, OMDS and the CSF marker levels in patients who discontinued treatment returned to pre-treatment levels. This rebound phenomenon was also observed in patients who discontinued oral prednisolone therapy independently of pulse therapy. Our findings suggest that CSF CXCL10 may serve as a therapy-response and therapy-predictive marker for HAM/TSP. In addition, since decrease in CSF CXCL10 level was associated with good functional prognosis, CSF CXCL10 is a potential surrogate marker for treatment of HAM/TSP.
Keywords: CXCL10; HAM/TSP; HTLV-1; biomarker; cerebrospinal fluid; neopterin.
Copyright © 2019 Tamaki, Sato, Tsugawa, Fujioka, Yagishita, Araya, Yamauchi, Coler-Reilly, Nagasaka, Hasegawa, Yamano and Tsuboi.
Figures


Similar articles
-
Multicenter, randomized, double-blind, placebo-controlled phase 3 study of mogamulizumab with open-label extension study in a minimum number of patients with human T-cell leukemia virus type-1-associated myelopathy.J Neurol. 2024 Jun;271(6):3471-3485. doi: 10.1007/s00415-024-12239-x. Epub 2024 Mar 2. J Neurol. 2024. PMID: 38430272 Free PMC article. Clinical Trial.
-
CSF CXCL10, CXCL9, and neopterin as candidate prognostic biomarkers for HTLV-1-associated myelopathy/tropical spastic paraparesis.PLoS Negl Trop Dis. 2013 Oct 10;7(10):e2479. doi: 10.1371/journal.pntd.0002479. eCollection 2013. PLoS Negl Trop Dis. 2013. PMID: 24130912 Free PMC article.
-
Following the Clues: Usefulness of Biomarkers of Neuroinflammation and Neurodegeneration in the Investigation of HTLV-1-Associated Myelopathy Progression.Front Immunol. 2021 Oct 26;12:737941. doi: 10.3389/fimmu.2021.737941. eCollection 2021. Front Immunol. 2021. PMID: 34764955 Free PMC article.
-
An update on human T-cell leukemia virus type I (HTLV-1)-associated myelopathy/tropical spastic paraparesis (HAM/TSP) focusing on clinical and laboratory biomarkers.Pharmacol Ther. 2021 Feb;218:107669. doi: 10.1016/j.pharmthera.2020.107669. Epub 2020 Aug 21. Pharmacol Ther. 2021. PMID: 32835825 Review.
-
Tropical spastic paraparesis and HTLV-1 associated myelopathy: clinical, epidemiological, virological and therapeutic aspects.Rev Neurol (Paris). 2012 Mar;168(3):257-69. doi: 10.1016/j.neurol.2011.12.006. Epub 2012 Mar 7. Rev Neurol (Paris). 2012. PMID: 22405461 Review.
Cited by
-
Multicenter, randomized, double-blind, placebo-controlled phase 3 study of mogamulizumab with open-label extension study in a minimum number of patients with human T-cell leukemia virus type-1-associated myelopathy.J Neurol. 2024 Jun;271(6):3471-3485. doi: 10.1007/s00415-024-12239-x. Epub 2024 Mar 2. J Neurol. 2024. PMID: 38430272 Free PMC article. Clinical Trial.
-
Pathogenesis of HTLV-1 infection and progression biomarkers: An overview.Braz J Infect Dis. 2021 May-Jun;25(3):101594. doi: 10.1016/j.bjid.2021.101594. Epub 2021 Jul 10. Braz J Infect Dis. 2021. PMID: 34256025 Free PMC article. Review.
-
Efficacy of Corticosteroid Therapy for HTLV-1-Associated Myelopathy: A Randomized Controlled Trial (HAMLET-P).Viruses. 2022 Jan 12;14(1):136. doi: 10.3390/v14010136. Viruses. 2022. PMID: 35062340 Free PMC article. Clinical Trial.
-
Systemic cytokines and GlycA discriminate disease status and predict corticosteroid response in HTLV-1-associated neuroinflammation.J Neuroinflammation. 2022 Dec 8;19(1):293. doi: 10.1186/s12974-022-02658-w. J Neuroinflammation. 2022. PMID: 36482436 Free PMC article.
-
Editorial: Biomarkers for prognosis of neuroinflammation and neurodegeneration associated with acute and chronic viral diseases.Front Neurosci. 2024 Jan 16;18:1354409. doi: 10.3389/fnins.2024.1354409. eCollection 2024. Front Neurosci. 2024. PMID: 38292447 Free PMC article. No abstract available.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

