Highly Efficient Preparation of Cyclic Dinucleotides via Engineering of Dinucleotide Cyclases in Escherichia coli
- PMID: 31572324
- PMCID: PMC6753226
- DOI: 10.3389/fmicb.2019.02111
Highly Efficient Preparation of Cyclic Dinucleotides via Engineering of Dinucleotide Cyclases in Escherichia coli
Abstract
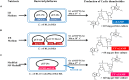
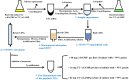
Cyclic dinucleotides (CDNs) are widely used secondary signaling molecules in bacterial and mammalian cells. The family of CDNs includes c-di-GMP, c-di-AMP and two distinct versions of hybrid cGAMPs. Studies related to these CDNs require large doses that are relatively expensive to generate by current methods. Here we report what to our knowledge is the first feasible microbial-based method to prepare these CDNs including c-di-GMP, 3'3'-cGAMP and 2'3'-cGAMP. The method mainly includes two parts: producing high yield of CDNs by engineering the overexpression of the proper dinucleotide cyclases (DNCs) and other related proteins in Escherichia coli, and purifying the bacteria-produced CDNs by a unified and simple process involving a STING affinity column, macroporous adsorption resin and C18 reverse-phase liquid chromatography. After purification, we obtained the diammonium salts of c-di-GMP, 3'3'-cGAMP and 2'3'-cGAMP with weight purity of >99, >96, >99% and in yields of >68, >26, and >82 milligrams per liter of culture, respectively. This technological platform enables the production of CDNs from cheaper material, provides a sustainable source of CDNs for scientific investigation, and can easily be further developed to prepare CDNs on a large scale for industry.
Keywords: adjuvant; affinity purification; cyclic dinucleotides; dinucleotide cyclases; microbial preparation.
Copyright © 2019 Lv, Sun, Wang, Lu, Li, Yuan, Zhu and Zhu.
Figures


Similar articles
-
Mass spectrometric characterization of cyclic dinucleotides (CDNs) in vivo.Anal Bioanal Chem. 2021 Nov;413(26):6457-6468. doi: 10.1007/s00216-021-03628-6. Epub 2021 Sep 2. Anal Bioanal Chem. 2021. PMID: 34476522 Free PMC article.
-
Chemical synthesis, purification, and characterization of 3'-5'-linked canonical cyclic dinucleotides (CDNs).Methods Enzymol. 2019;625:41-59. doi: 10.1016/bs.mie.2019.04.022. Epub 2019 May 20. Methods Enzymol. 2019. PMID: 31455536
-
Distinct Dynamic and Conformational Features of Human STING in Response to 2'3'-cGAMP and c-di-GMP.Chembiochem. 2019 Jul 15;20(14):1838-1847. doi: 10.1002/cbic.201900051. Epub 2019 Jun 5. Chembiochem. 2019. PMID: 30895657
-
Biotechnological production of cyclic dinucleotides-Challenges and opportunities.Biotechnol Bioeng. 2022 Mar;119(3):677-684. doi: 10.1002/bit.28027. Epub 2022 Jan 4. Biotechnol Bioeng. 2022. PMID: 34953086 Review.
-
Cyclic di-GMP: second messenger extraordinaire.Nat Rev Microbiol. 2017 May;15(5):271-284. doi: 10.1038/nrmicro.2016.190. Epub 2017 Feb 6. Nat Rev Microbiol. 2017. PMID: 28163311 Review.
Cited by
-
Advances in the Synthesis and Analysis of Biologically Active Phosphometabolites.Int J Mol Sci. 2023 Feb 5;24(4):3150. doi: 10.3390/ijms24043150. Int J Mol Sci. 2023. PMID: 36834560 Free PMC article. Review.
-
The efficient synthesis and purification of 2'3'- cGAMP from Escherichia coli.Front Microbiol. 2024 Mar 8;15:1345617. doi: 10.3389/fmicb.2024.1345617. eCollection 2024. Front Microbiol. 2024. PMID: 38525075 Free PMC article.
-
Enzymatic Syntheses and Applications of Fluorescent Cyclic Dinucleotides.Chemistry. 2020 May 12;26(27):6076-6084. doi: 10.1002/chem.202001194. Epub 2020 Apr 28. Chemistry. 2020. PMID: 32157755 Free PMC article.
-
Advancements in enzymatic reaction-mediated microbial transformation.Heliyon. 2024 Sep 20;10(19):e38187. doi: 10.1016/j.heliyon.2024.e38187. eCollection 2024 Oct 15. Heliyon. 2024. PMID: 39430465 Free PMC article. Review.
-
Enhanced Immune Responses in Mice Induced by the c-di-GMP Adjuvanted Inactivated Vaccine for Pseudorabies Virus.Front Immunol. 2022 Mar 31;13:845680. doi: 10.3389/fimmu.2022.845680. eCollection 2022. Front Immunol. 2022. PMID: 35432301 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

