Two New 1,3,4-Oxadiazoles With Effective Antifungal Activity Against Candida albicans
- PMID: 31572335
- PMCID: PMC6751290
- DOI: 10.3389/fmicb.2019.02130
Two New 1,3,4-Oxadiazoles With Effective Antifungal Activity Against Candida albicans
Abstract
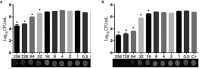
Candida infections have become a serious public health problem with high mortality rates, especially in immunocompromised patients, since Candida albicans is the major opportunistic pathogen responsible for systemic or invasive candidiasis. Commercially available antifungal agents are restricted and fungal resistance to such drugs has increased; therefore, the development of a more specific antifungal agent is necessary. Using assays for antifungal activity, here we report that two new compounds of 1,3,4-oxadiazoles class (LMM5 and LMM11), which were discovered by in silico methodologies as possible thioredoxin reductase inhibitors, were effective against C. albicans. Both compounds had in vitro antifungal activity with MIC 32 μg/ml. Cytotoxicity in vitro demonstrated that LMM5 and LMM11 were non-toxic in the cell lines evaluated. The kinetic of the time-kill curve suggested a fungistatic profile and showed an inhibitory effect of LMM5 and LMM11 in 12 h that remained for 24 and 36 h, which is better than fluconazole. In the murine systemic candidiasis model by C. albicans, the two compounds significantly reduced the renal and spleen fungal burden. According to the SEM and TEM images, we hypothesize that the mechanism of action of LMM5 and LMM11 is directly related to the inhibition of the enzyme thioredoxin reductase and internally affect the fungal cell. In view of all in vitro and in vivo results, LMM5 and LMM11 are effective therapeutic candidates for the development of new antifungal drugs addressing the treatment of human infections caused by C. albicans.
Keywords: Candida albicans; antifungal activity; in vitro; in vivo; thioredoxin reductase.
Copyright © 2019 Capoci, Sakita, Faria, Rodrigues-Vendramini, Arita, de Oliveira, Felipe, Maigret, Bonfim-Mendonça, Kioshima and Svidzinski.
Figures




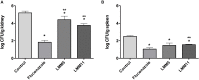

Similar articles
-
Promising antifungal activity of new oxadiazole against Candida krusei.PLoS One. 2020 Jan 14;15(1):e0227876. doi: 10.1371/journal.pone.0227876. eCollection 2020. PLoS One. 2020. PMID: 31935275 Free PMC article.
-
Candida and candidaemia. Susceptibility and epidemiology.Dan Med J. 2013 Nov;60(11):B4698. Dan Med J. 2013. PMID: 24192246 Review.
-
β-lactam substituted polycyclic fused pyrrolidine/pyrrolizidine derivatives eradicate C. albicans in an ex vivo human dentinal tubule model by inhibiting sterol 14-α demethylase and cAMP pathway.Biochim Biophys Acta. 2016 Apr;1860(4):636-47. doi: 10.1016/j.bbagen.2015.12.020. Epub 2015 Dec 23. Biochim Biophys Acta. 2016. PMID: 26723175
-
Antifungal Activity and Potential Mechanism of N-Butylphthalide Alone and in Combination With Fluconazole Against Candida albicans.Front Microbiol. 2019 Jul 2;10:1461. doi: 10.3389/fmicb.2019.01461. eCollection 2019. Front Microbiol. 2019. PMID: 31312187 Free PMC article.
-
Combination of fluconazole with non-antifungal agents: a promising approach to cope with resistant Candida albicans infections and insight into new antifungal agent discovery.Int J Antimicrob Agents. 2014 May;43(5):395-402. doi: 10.1016/j.ijantimicag.2013.12.009. Epub 2014 Jan 22. Int J Antimicrob Agents. 2014. PMID: 24503221 Review.
Cited by
-
2-Substituted-3-(5-Substituted-1,3,4-oxadiazol/thiadiazol-2-yl) Thiazolidin-4-one Derivatives: Synthesis, Anticancer, Antimicrobial, and Antioxidant Potential.Pharmaceuticals (Basel). 2023 May 29;16(6):805. doi: 10.3390/ph16060805. Pharmaceuticals (Basel). 2023. PMID: 37375752 Free PMC article.
-
Promising antifungal activity of new oxadiazole against Candida krusei.PLoS One. 2020 Jan 14;15(1):e0227876. doi: 10.1371/journal.pone.0227876. eCollection 2020. PLoS One. 2020. PMID: 31935275 Free PMC article.
-
Overview of Antifungal Drugs against Paracoccidioidomycosis: How Do We Start, Where Are We, and Where Are We Going?J Fungi (Basel). 2020 Nov 19;6(4):300. doi: 10.3390/jof6040300. J Fungi (Basel). 2020. PMID: 33228010 Free PMC article. Review.
-
Synthesis, crystal structure and Hirshfeld surface analysis of a zinc(II) coordination polymer of 5-phenyl-1,3,4-oxa-diazole-2-thiol-ate.Acta Crystallogr E Crystallogr Commun. 2022 Jul 14;78(Pt 8):794-797. doi: 10.1107/S2056989022006922. eCollection 2022 Aug 1. Acta Crystallogr E Crystallogr Commun. 2022. PMID: 35974814 Free PMC article.
-
Fungicidal Activity of a Safe 1,3,4-Oxadiazole Derivative Against Candida albicans.Pathogens. 2021 Mar 7;10(3):314. doi: 10.3390/pathogens10030314. Pathogens. 2021. PMID: 33800117 Free PMC article.
References
LinkOut - more resources
Full Text Sources

