Trypanosoma brucei Secreted Aromatic Ketoacids Activate the Nrf2/HO-1 Pathway and Suppress Pro-inflammatory Responses in Primary Murine Glia and Macrophages
- PMID: 31572363
- PMCID: PMC6749089
- DOI: 10.3389/fimmu.2019.02137
Trypanosoma brucei Secreted Aromatic Ketoacids Activate the Nrf2/HO-1 Pathway and Suppress Pro-inflammatory Responses in Primary Murine Glia and Macrophages
Abstract
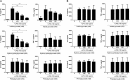
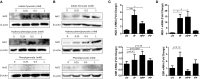
African trypanosomes, such as Trypanosoma brucei (T. brucei), are protozoan parasites of the mammalian vasculature and central nervous system that are best known for causing fatal human sleeping sickness. As exclusively extracellular parasites, trypanosomes are subject to constant challenge from host immune defenses but they have developed very effective strategies to evade and modulate these responses to maintain an infection while simultaneously prolonging host survival. Here we investigate host parasite interactions, especially within the CNS context, which are not well-understood. We demonstrate that T. brucei strongly upregulates the stress response protein, Heme Oxygenase 1 (HO-1), in primary murine glia and macrophages in vitro. Furthermore, using a novel AHADHinT. brucei cell line, we demonstrate that specific aromatic ketoacids secreted by bloodstream forms of T. brucei are potent drivers of HO-1 expression and are capable of inhibiting pro-IL1β induction in both glia and macrophages. Additionally, we found that these ketoacids significantly reduced IL-6 and TNFα production by glia, but not macrophages. Finally, we present data to support Nrf2 activation as the mechanism of action by which these ketoacids upregulate HO-1 expression and mediate their anti-inflammatory activity. This study therefore reports a novel immune evasion mechanism, whereby T. brucei secretes amino-acid derived metabolites for the purpose of suppressing both the host CNS and peripheral immune response, potentially via induction of the Nrf2/HO-1 pathway.
Keywords: glia; immune suppression; keto acids; macrophages; trypanosomes.
Copyright © 2019 Campbell, Williams, Fitzgerald, Barry, Cunningham, Nolan and Dunne.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

