Factors Affecting Early Antibody Secreting Cell Maturation Into Long-Lived Plasma Cells
- PMID: 31572364
- PMCID: PMC6749102
- DOI: 10.3389/fimmu.2019.02138
Factors Affecting Early Antibody Secreting Cell Maturation Into Long-Lived Plasma Cells
Abstract
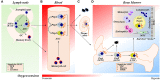
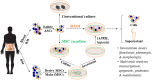
Antibody secreting cells (ASCs) are terminally differentiated cells of the humoral immune response and must adapt morphologically, transcriptionally, and metabolically to maintain high-rates of antibody (Ab) secretion. ASCs differentiate from activated B cells in lymph nodes and transiently circulate in the blood. Most of the circulating ASCs undergo apoptosis, but a small fraction of early ASCs migrate to the bone marrow (BM) and eventually mature into long-lived plasma cells (LLPCs). LLPC survival is controlled both intrinsically and extrinsically. Their differentiation and maintenance programs are governed by many intrinsic mechanisms involving anti-apoptosis, autophagy, and metabolism. The extrinsic factors involved in LLPC generation include BM stromal cells, cytokines, and chemokines, such as APRIL, IL-6, and CXCL12. In humans, the BM CD19-CD38hiCD138+ ASC subset is the main repository of LLPCs, and our recent development of an in vitro BM mimic provides essential tools to study environmental cues that support LLPC survival and the critical molecular mechanisms of maturation from early minted blood ASCs to LLPCs. In this review, we summarize the evidence of LLPC generation and maintenance and provide novel paradigms of LLPC maturation.
Keywords: B cell; antibody-secreting cell; differentiation; immunoglobulin; long-lived plasma cell; maintenance; maturation.
Copyright © 2019 Nguyen, Joyner, Sanz and Lee.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

