Neutrophils as Suppressors of T Cell Proliferation: Does Age Matter?
- PMID: 31572368
- PMCID: PMC6749034
- DOI: 10.3389/fimmu.2019.02144
Neutrophils as Suppressors of T Cell Proliferation: Does Age Matter?
Abstract
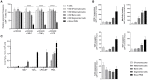
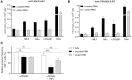
Whereas, neutrophils have long been considered to mainly function as efficient innate immunity killers of micro-organisms at infected sites, they are now recognized to also be involved in modulation of adaptive immune responses. Immature and mature neutrophils were reported to have the capacity to suppress T cell-mediated immune responses as so-called granulocyte-myeloid-derived suppressor cells (g-MDSCs), and thereby affect the clinical outcome of cancer patients and impact the chronicity of microbial infections or rejection reactions in organ transplantation settings. These MDSCs were at first considered to be immature myeloid cells that left the bone marrow due to disease-specific signals. Current studies show that also mature neutrophils can exert suppressive activity. In this study we investigated in a robust T cell suppression assay whether immature CD11b+ myeloid cells were capable of MDSC activity comparable to mature fully differentiated neutrophils. We compared circulating neutrophils with myeloid cell fractions from the bone marrow at different differentiation stages. Our results indicate that functional MDSC activity is only becoming detectable at the final stage of differentiation, depending on the procedure of cell isolation. The MDSC activity obtained during neutrophil maturation correlated with the induction of the well-known highly mobile and toxic effector functions of the circulating neutrophil. Although immature neutrophils have been suggested to be increased in the circulation of cancer patients, we show here that immature neutrophils are not efficient in suppressing T cells. This suggests that the presence of immature neutrophils in the bloodstream of cancer patients represent a mere association or may function as a source of mature neutrophils in the tumor environment but not a direct cause of enhanced MDSC activity in cancer.
Keywords: MDSC activity; T cell suppression; cell isolation; neutrophils; progenitors.
Copyright © 2019 Aarts, Hiemstra, Tool, van den Berg, Mul, van Bruggen and Kuijpers.
Figures


References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

