Lovastatin Inhibits HIV-1-Induced MHC-I Downregulation by Targeting Nef-AP-1 Complex Formation: A New Strategy to Boost Immune Eradication of HIV-1 Infected Cells
- PMID: 31572371
- PMCID: PMC6749138
- DOI: 10.3389/fimmu.2019.02151
Lovastatin Inhibits HIV-1-Induced MHC-I Downregulation by Targeting Nef-AP-1 Complex Formation: A New Strategy to Boost Immune Eradication of HIV-1 Infected Cells
Abstract
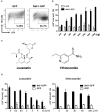
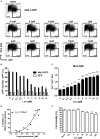
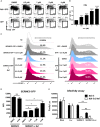
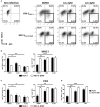
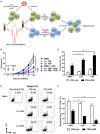
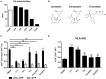
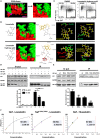
Current combined antiretroviral therapy (cART) mainly targets 3 of the 15 HIV proteins leaving many potential viral vulnerabilities unexploited. To purge the HIV-1 latent reservoir, various strategies including "shock and kill" have been developed. A key question is how to restore impaired immune surveillance. HIV-1 protein Nef has long been known to mediate the downregulation of cell-surface MHC-I and assist HIV-1 to evade the immune system. Through high throughput screening of Food and Drug Administration (FDA) approved drugs, we identified lovastatin, a statin drug, to significantly antagonize Nef to downregulate MHC-I, CD4, and SERINC5, and inhibit the intrinsic infectivity of virions. In addition, lovastatin boosted autologous CTLs to eradicate the infected cells and effectively inhibit the subsequent viral rebound in CD4+ T-lymphocytes isolated from HIV-1-infected individuals receiving suppressive cART. Furthermore, we found that lovastatin inhibits Nef-induced MHC-I downregulation by directly binding with Nef and disrupting the Nef-AP-1 complex. These results demonstrate that lovastatin is a promising agent for counteracting Nef-mediated downregulation of MHC-I, CD4, and SERINC5. Lovastatin could potentially be used in the clinic to enhance anti-HIV-1 immune surveillance.
Keywords: AP-1; CD4; HIV-1; MHC-I; Nef; SERINC5; immune surveillance; lovastatin.
Copyright © 2019 Liu, Zhang, Zhang, Wu, Jing, Liu, Xia, Zou, Lu, Ma, He, Hu, Zhang, Deng, Cai, Tang, Peng, Zhang and Li.
Figures
Similar articles
-
An Amino Acid Polymorphism within the HIV-1 Nef Dileucine Motif Functionally Uncouples Cell Surface CD4 and SERINC5 Downregulation.J Virol. 2021 Jul 26;95(16):e0058821. doi: 10.1128/JVI.00588-21. Epub 2021 Jul 26. J Virol. 2021. PMID: 34037423 Free PMC article.
-
HIV Subtype and Nef-Mediated Immune Evasion Function Correlate with Viral Reservoir Size in Early-Treated Individuals.J Virol. 2019 Mar 5;93(6):e01832-18. doi: 10.1128/JVI.01832-18. Print 2019 Mar 15. J Virol. 2019. PMID: 30602611 Free PMC article.
-
Association between HIV-1 Nef-mediated MHC-I downregulation and the maintenance of the replication-competent latent viral reservoir in individuals with virally suppressed HIV-1 in Uganda: an exploratory cohort study.Lancet Microbe. 2025 May;6(5):101018. doi: 10.1016/j.lanmic.2024.101018. Epub 2025 Mar 12. Lancet Microbe. 2025. PMID: 40088911 Free PMC article.
-
Structure, function, and inhibitor targeting of HIV-1 Nef-effector kinase complexes.J Biol Chem. 2020 Oct 30;295(44):15158-15171. doi: 10.1074/jbc.REV120.012317. Epub 2020 Aug 29. J Biol Chem. 2020. PMID: 32862141 Free PMC article. Review.
-
The downregulation of CD4 and MHC-I by primate lentiviruses: a paradigm for the modulation of cell surface receptors.Immunol Rev. 1999 Apr;168:51-63. doi: 10.1111/j.1600-065x.1999.tb01282.x. Immunol Rev. 1999. PMID: 10399064 Review.
Cited by
-
Antiretroviral Drug Discovery Targeting the HIV-1 Nef Virulence Factor.Viruses. 2022 Sep 13;14(9):2025. doi: 10.3390/v14092025. Viruses. 2022. PMID: 36146831 Free PMC article. Review.
-
Computational approach to decipher cellular interactors and drug targets during co-infection of SARS-CoV-2, Dengue, and Chikungunya virus.Virusdisease. 2021 Mar;32(1):55-64. doi: 10.1007/s13337-021-00665-8. Epub 2021 Mar 10. Virusdisease. 2021. PMID: 33723515 Free PMC article.
-
Structure-Activity Relationships of Natural and Semisynthetic Plecomacrolides Suggest Distinct Pathways for HIV-1 Immune Evasion and Vacuolar ATPase-Dependent Lysosomal Acidification.J Med Chem. 2024 Mar 28;67(6):4483-4495. doi: 10.1021/acs.jmedchem.3c01574. Epub 2024 Mar 7. J Med Chem. 2024. PMID: 38452116 Free PMC article.
-
The HIV-1 proviral landscape reveals that Nef contributes to HIV-1 persistence in effector memory CD4+ T cells.J Clin Invest. 2022 Apr 1;132(7):e154422. doi: 10.1172/JCI154422. J Clin Invest. 2022. PMID: 35133986 Free PMC article.
-
The ORF8 protein of SARS-CoV-2 mediates immune evasion through down-regulating MHC-Ι.Proc Natl Acad Sci U S A. 2021 Jun 8;118(23):e2024202118. doi: 10.1073/pnas.2024202118. Proc Natl Acad Sci U S A. 2021. PMID: 34021074 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

