Schistosoma japonicum Infection Promotes the Response of Tfh Cells Through Down-Regulation of Caspase-3-Mediating Apoptosis
- PMID: 31572373
- PMCID: PMC6753327
- DOI: 10.3389/fimmu.2019.02154
Schistosoma japonicum Infection Promotes the Response of Tfh Cells Through Down-Regulation of Caspase-3-Mediating Apoptosis
Abstract
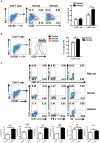
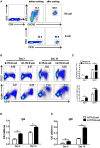
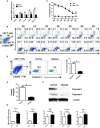
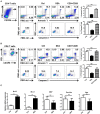
CD4+ T follicular helper (Tfh) cells, a new subset of immune cells, have been demonstrated to be involved in granulomatous responses to Schistosoma japonicum (S. japonicum) infection. However, the role and underlying mechanisms of Tfh cell aggregation in S. japonicum infection remain incompletely understood. In this study, we provide evidence that S. japonicum infection enhances the accumulation of Tfh cells in the spleen, lymph nodes, and peripheral blood of C57BL/6 mice. Infection-induced Tfh cells exhibited more potent effects directly on B cell responses than the control Tfh cells (P < 0.05). Furthermore, reduced apoptosis of Tfh cells was found both in S. japonicum infected mice and in soluble egg antigen (SEA) treated Tfh cells (P < 0.05). Mechanistic studies reveal that caspase-3 is the primary drivers of down-regulated apoptotic Tfh cell death in S. japonicum infection. In summary, this study demonstrates that Tfh cell accumulation might have an impact on the generation of immune responses in S. japonicum infection, and caspase-3 signaling mediated apoptosis down-regulation might responsible for the accumulation of Tfh cell in this course.
Keywords: SEA; Schistosoma japonicum; T follicular helper (Tfh) cells; apoptosis; caspase-3.
Copyright © 2019 Yang, Qu, Jin, Feng, Xie, Zhu, Liu, Xie, Qiu, Qi, Mu and Huang.
Figures


Similar articles
-
Eggs of Schistosoma japonicum deposited in the spleen induce apoptosis of splenic T cells in C57BL/6 mice.Parasitol Res. 2025 Mar 10;124(3):31. doi: 10.1007/s00436-025-08474-4. Parasitol Res. 2025. PMID: 40059230 Free PMC article.
-
Ikzf2 Regulates the Development of ICOS+ Th Cells to Mediate Immune Response in the Spleen of S. japonicum-Infected C57BL/6 Mice.Front Immunol. 2021 Aug 12;12:687919. doi: 10.3389/fimmu.2021.687919. eCollection 2021. Front Immunol. 2021. PMID: 34475870 Free PMC article.
-
Granulocytic myeloid-derived suppressor cells inhibit T follicular helper cells during experimental Schistosoma japonicum infection.Parasit Vectors. 2021 Sep 26;14(1):497. doi: 10.1186/s13071-021-05006-8. Parasit Vectors. 2021. PMID: 34565440 Free PMC article.
-
[Impact of gender on hepatic pathology and antibody - mediated immunity caused by Schistosoma japonicum infection in C57BL/6 mice].Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2020 May 7;32(3):255-261. doi: 10.16250/j.32.1374.2020010. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. 2020. PMID: 32468787 Chinese.
-
Schistosoma japonicum Associated Colorectal Cancer and Its Management.Acta Parasitol. 2023 Dec;68(4):723-734. doi: 10.1007/s11686-023-00707-9. Epub 2023 Aug 18. Acta Parasitol. 2023. PMID: 37594685 Review.
Cited by
-
Eggs of Schistosoma japonicum deposited in the spleen induce apoptosis of splenic T cells in C57BL/6 mice.Parasitol Res. 2025 Mar 10;124(3):31. doi: 10.1007/s00436-025-08474-4. Parasitol Res. 2025. PMID: 40059230 Free PMC article.
-
Inhibition of COX-2 ameliorates murine liver schistosomiasis japonica through splenic cellular immunoregulation.Parasit Vectors. 2022 Apr 23;15(1):144. doi: 10.1186/s13071-022-05201-1. Parasit Vectors. 2022. PMID: 35461268 Free PMC article.
-
The Protective Effect of the Soluble Egg Antigen of Schistosoma japonicum in A Mouse Skin Transplantation Model.Front Immunol. 2022 Jul 14;13:884006. doi: 10.3389/fimmu.2022.884006. eCollection 2022. Front Immunol. 2022. PMID: 35911717 Free PMC article.
-
Schistosoma japonicum Infection in Treg-Specific USP21 Knockout Mice.J Immunol Res. 2021 Feb 9;2021:6613162. doi: 10.1155/2021/6613162. eCollection 2021. J Immunol Res. 2021. PMID: 33628844 Free PMC article.
-
Plantaricin BM-1 decreases viability of SW480 human colorectal cancer cells by inducing caspase-dependent apoptosis.Front Microbiol. 2023 Jan 4;13:1103600. doi: 10.3389/fmicb.2022.1103600. eCollection 2022. Front Microbiol. 2023. PMID: 36687624 Free PMC article.
References
-
- Zhang Y, Zhang J, Bo SY, Wang GZ, Xin XF. Observation on dynamic changes of SEA specific antibody in sera of BALB/c mice infected with Schistosoma japonicum. Zhongguo Xue Xi Chong Bing Fang Zhi Za Zhi. (2012) 24:284–89. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

