Autoantibodies to Killer Cell Immunoglobulin-Like Receptors in Patients With Systemic Lupus Erythematosus Induce Natural Killer Cell Hyporesponsiveness
- PMID: 31572377
- PMCID: PMC6749077
- DOI: 10.3389/fimmu.2019.02164
Autoantibodies to Killer Cell Immunoglobulin-Like Receptors in Patients With Systemic Lupus Erythematosus Induce Natural Killer Cell Hyporesponsiveness
Abstract
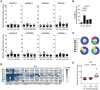
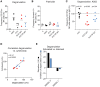
Natural killer (NK) cell cytotoxicity toward self-cells is restrained by the inhibitory HLA class I-binding receptors CD94/NKG2A and the killer cell immunoglobulin-like receptors (KIRs). CD94/NKG2A and KIRs are also essential for NK cell education, which is a dynamic functional maturation process where a constitutive binding of inhibitory receptors to cognate HLA class I molecules is required for NK cells to maintain their full cytotoxic capacity. Previously, we described autoantibodies to CD94/NKG2A in patients with systemic lupus erythematosus (SLE). In this study we analyzed sera from 191 patients with SLE, 119 patients with primary Sjögren's syndrome (pSS), 48 patients with systemic sclerosis (SSc), and 100 healthy donors (HD) for autoantibodies to eight different KIRs. Anti-KIR autoantibodies were identified in sera from 23.0% of patients with SLE, 10.9% of patients with pSS, 12.5% of patients with SSc, and 3.0% of HD. IgG from anti-KIR-positive SLE patients reduced the degranulation and cytotoxicity of NK cells toward K562 tumor cells. The presence of anti-KIR-autoantibodies reacting with >3 KIRs was associated with an increased disease activity (p < 0.0001), elevated serum levels of IFN-α (p < 0.0001), nephritis (p = 0.001), and the presence of anti-Sm (p = 0.007), and anti-RNP (p = 0.003) autoantibodies in serum. Together these findings suggest that anti-KIR autoantibodies may contribute to the reduced function of NK cells in SLE patients, and that a defective NK cell function may be a risk factor for the development of lupus nephritis.
Keywords: autoantibody; killer cell immunoglobulin-like receptor; natural killer cells; nephritis; primary Sjögren's syndrome; systemic lupus erythematosus.
Copyright © 2019 Segerberg, Lundtoft, Reid, Hjorton, Leonard, Nordmark, Carlsten and Hagberg.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

