The Involvement and Therapy Target of Immune Cells After Ischemic Stroke
- PMID: 31572378
- PMCID: PMC6749156
- DOI: 10.3389/fimmu.2019.02167
The Involvement and Therapy Target of Immune Cells After Ischemic Stroke
Abstract
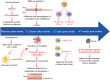
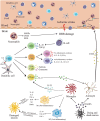
After ischemic stroke, the integrity of the blood-brain barrier is compromised. Peripheral immune cells, including neutrophils, T cells, B cells, dendritic cells, and macrophages, infiltrate into the ischemic brain tissue and play an important role in regulating the progression of ischemic brain injury. In this review, we will discuss the role of different immune cells after stroke in the secondary inflammatory reaction and focus on the phenotypes and functions of macrophages in ischemic stroke, as well as briefly introduce the anti-ischemic stroke therapy targeting macrophages.
Keywords: immune cell; inflammation; ischemic stroke; macrophage; microglia.
Copyright © 2019 Jian, Liu, Zhu, Smerin, Zhong, Gu, Fang and Xiong.
Figures

References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

