Innate Immune Evasion of Alphaherpesvirus Tegument Proteins
- PMID: 31572398
- PMCID: PMC6753173
- DOI: 10.3389/fimmu.2019.02196
Innate Immune Evasion of Alphaherpesvirus Tegument Proteins
Abstract
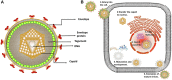
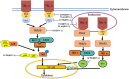
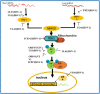
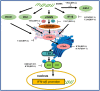
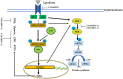
Alphaherpesviruses are a large family of highly successful human and animal DNA viruses that can establish lifelong latent infection in neurons. All alphaherpesviruses have a protein-rich layer called the tegument that, connects the DNA-containing capsid to the envelope. Tegument proteins have a variety of functions, playing roles in viral entry, secondary envelopment, viral capsid nuclear transportation during infection, and immune evasion. Recently, many studies have made substantial breakthroughs in characterizing the innate immune evasion of tegument proteins. A wide range of antiviral tegument protein factors that control incoming infectious pathogens are induced by the type I interferon (IFN) signaling pathway and other innate immune responses. In this review, we discuss the immune evasion of tegument proteins with a focus on herpes simplex virus type I.
Keywords: IFN; alphaherpesvirus; immune evasion; signaling pathway; tegument protein.
Copyright © 2019 Yang, Wang, Cheng, Yang, Wu, Jia, Liu, Zhu, Chen, Zhang, Zhao, Huang, Wang, Xu, Chen, Zhu, Luo, Liu, Yu, Zhang, Tian, Pan, Rehman and Chen.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

