The Era of Thromboinflammation: Platelets Are Dynamic Sensors and Effector Cells During Infectious Diseases
- PMID: 31572400
- PMCID: PMC6753373
- DOI: 10.3389/fimmu.2019.02204
The Era of Thromboinflammation: Platelets Are Dynamic Sensors and Effector Cells During Infectious Diseases
Abstract
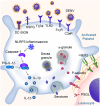
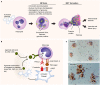
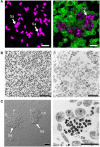
Platelets are anucleate cells produced by megakaryocytes. In recent years, a robust body of literature supports the evolving role of platelets as key sentinel and effector cells in infectious diseases, especially critical in bridging hemostatic, inflammatory, and immune continuums. Upon intravascular pathogen invasion, platelets can directly sense viral, parasitic, and bacterial infections through pattern recognition receptors and integrin receptors or pathogen: immunoglobulin complexes through Fc and complement receptors-although our understanding of these interactions remains incomplete. Constantly scanning for areas of injury or inflammation as they circulate in the vasculature, platelets also indirectly respond to pathogen invasion through interactions with leukocytes and the endothelium. Following antigen recognition, platelets often become activated. Through a diverse repertoire of mechanisms, activated platelets can directly sequester or kill pathogens, or facilitate pathogen clearance by activating macrophages and neutrophils, promoting neutrophil extracellular traps (NETs) formation, forming platelet aggregates and microthrombi. At times, however, platelet activation may also be injurious to the host, exacerbating inflammation and promoting endothelial damage and thrombosis. There are many gaps in our understandings of the role of platelets in infectious diseases. However, with the emergence of advanced technologies, our knowledge is increasing. In the current review, we mainly discuss these evolving roles of platelets under four different infectious pathogen infections, of which are dengue, malaria, Esterichia coli (E. coli) and staphylococcus aureus S. aureus, highlighting the complex interplay of these processes with hemostatic and thrombotic pathways.
Keywords: bacteria; dengue; infection; inflammation; malaria; platelet; sepsis; thromboinflammation.
Copyright © 2019 Guo and Rondina.
Figures

References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

