Neuropilin-1 Acts as a Receptor for Complement Split Products
- PMID: 31572401
- PMCID: PMC6753332
- DOI: 10.3389/fimmu.2019.02209
Neuropilin-1 Acts as a Receptor for Complement Split Products
Abstract
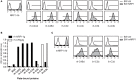
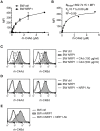
Complement split products (CSPs), such as the fragments C4d and C3d, which are generated as a consequence of complement regulatory processes, are established markers for disease activity in autoimmunity or antibody-mediated graft rejection. Since immunoglobulin-like transcript 4 (ILT4) was previously shown to interact with soluble CSPs, but not with CSPs covalently-bound to target surfaces following classical complement activation, the present study aimed to identify novel cellular receptors interacting with covalently-deposited CSPs. By applying an unbiased screening approach using a cDNA mammalian expression library generated from human monocyte-derived dendritic cells and probed with recombinant human C4d, we identified neuropilin-1 (NRP1) as a novel receptor for C4d, C3d, and iC3b. NRP1, a highly conserved type 1 transmembrane protein, plays important roles in the development of the nervous and cardiovascular system as well as in tumorigenesis through interaction with its established binding partners, such as vascular endothelial growth factor (VEGF) and semaphorin 3A (Sema3A). NRP1 is also expressed on immune cells and serves as a marker for murine Tregs. Although NRP1 contains domains homologous to ones found in some complement proteins, it has not been linked to the complement system. We demonstrate that binding of C4d to NRP1 expressing cells was dose-dependent and saturable, and had a KD value of 0.71 μM. Importantly, and in contrast to ILT4, NRP1 interacted with CSPs that were covalently bound to target surfaces in the course of complement activation, therefore representing a classical complement receptor. The binding site of CSPs was mapped to the b1 domain of the coagulation factor V/VIII homology domain of NRP1. Taken together, our results demonstrate a novel role for NRP1 as a receptor for CSPs deposited on surfaces during complement activation. Further work is required to elucidate the functional consequences of the NRP1-CSP interactions in immunity.
Keywords: C3d; C4d; complement receptors; complement split products; iC3b; neuropilin-1.
Copyright © 2019 Battin, De Sousa Linhares, Paster, Isenman, Wahrmann, Leitner, Zlabinger, Steinberger and Hofer.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

