Vitamin D Signaling in the Context of Innate Immunity: Focus on Human Monocytes
- PMID: 31572402
- PMCID: PMC6753645
- DOI: 10.3389/fimmu.2019.02211
Vitamin D Signaling in the Context of Innate Immunity: Focus on Human Monocytes
Abstract
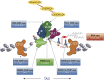
The vitamin D3 metabolite 1α,25-dihydroxyvitamin D3 (1,25(OH)2D3) activates at sub-nanomolar concentrations the transcription factor vitamin D receptor (VDR). VDR is primarily involved in the control of cellular metabolism but in addition modulates processes important for immunity, such as anti-microbial defense and the induction of T cell tolerance. Monocytes and their differentiated phenotypes, macrophages and dendritic cells, are key cell types of the innate immune system, in which vitamin D signaling was most comprehensively investigated via the use of next generation sequencing technologies. These investigations provided genome-wide maps illustrating significant effects of 1,25(OH)2D3 on the binding of VDR, the pioneer transcription factors purine-rich box 1 (PU.1) and CCAAT/enhancer binding protein α (CEBPA) and the chromatin modifier CCCTC-binding factor (CTCF) as well as on chromatin accessibility and histone markers of promoter and enhancer regions, H3K4me3 and H3K27ac. Thus, the epigenome of human monocytes is at multiple levels sensitive to vitamin D. These data served as the basis for the chromatin model of vitamin D signaling, which mechanistically explains the activation of a few hundred primary vitamin D target genes. Comparable epigenome- and transcriptome-wide effects of vitamin D were also described in peripheral blood mononuclear cells isolated from individuals before and after supplementation with a vitamin D3 bolus. This review will conclude with the hypothesis that vitamin D modulates the epigenome of immune cells during perturbations by antigens and other immunological challenges suggesting that an optimal vitamin D status may be essential for an effective epigenetic learning process, in particular of the innate immune system.
Keywords: PBMCs; VDR; epigenome; gene regulation; monocytes; transcriptome; vitamin D; vitamin D target genes.
Copyright © 2019 Carlberg.
Figures

References
-
- Holick MF. Photobiology of Vitamin D. Vitamin D, 3rd ed. (2011). p. 13–22.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

