Glucocorticoids and Glucocorticoid-Induced-Leucine-Zipper (GILZ) in Psoriasis
- PMID: 31572404
- PMCID: PMC6753639
- DOI: 10.3389/fimmu.2019.02220
Glucocorticoids and Glucocorticoid-Induced-Leucine-Zipper (GILZ) in Psoriasis
Abstract
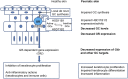
Psoriasis is a prevalent chronic inflammatory human disease initiated by impaired function of immune cells and epidermal keratinocytes, resulting in increased cytokine production and hyperproliferation, leading to skin lesions. Overproduction of Th1- and Th17-cytokines including interferon (IFN)-γ, tumor necrosis factor (TNF)-α, interleukin (IL)-23, IL-17, and IL-22, is a major driver of the disease. Glucocorticoids (GCs) represent the mainstay protocol for treating psoriasis as they modulate epidermal differentiation and are potent anti-inflammatory compounds. The development of safer GC-based therapies is a high priority due to potentially severe adverse effects associated with prolonged GC use. Specific efforts have focused on downstream anti-inflammatory effectors of GC-signaling such as GC-Induced-Leucine-Zipper (GILZ), which suppresses Th17 responses and antagonizes multiple pro-inflammatory signaling pathways involved in psoriasis, including AP-1, NF-κB, STAT3, and ROR-γt. Here we review evidence regarding defective GC signaling, GC receptor (GR) function, and GILZ in psoriasis. We discuss seemingly contradicting data on the loss- and gain-of-function of GILZ in the imiquimod-induced mouse model of psoriasis. We also present potential therapeutic strategies aimed to restore GC-related pathways.
Keywords: glucocorticoid-induced-leucine-zipper (GILZ/TSC22D3); glucocorticoids (GCs); immune cells; keratinocytes; psoriasis; signaling; skin inflammation.
Copyright © 2019 Sevilla and Pérez.
Figures

Similar articles
-
Overexpression of Glucocorticoid-induced Leucine Zipper (GILZ) increases susceptibility to Imiquimod-induced psoriasis and involves cutaneous activation of TGF-β1.Sci Rep. 2016 Dec 9;6:38825. doi: 10.1038/srep38825. Sci Rep. 2016. PMID: 27934944 Free PMC article.
-
GILZ regulates Th17 responses and restrains IL-17-mediated skin inflammation.J Autoimmun. 2015 Jul;61:73-80. doi: 10.1016/j.jaut.2015.05.010. Epub 2015 Jun 13. J Autoimmun. 2015. PMID: 26077873
-
Could GILZ Be the Answer to Glucocorticoid Toxicity in Lupus?Front Immunol. 2019 Jul 17;10:1684. doi: 10.3389/fimmu.2019.01684. eCollection 2019. Front Immunol. 2019. PMID: 31379872 Free PMC article. Review.
-
Implicating the Role of GILZ in Glucocorticoid Modulation of T-Cell Activation.Front Immunol. 2019 Aug 7;10:1823. doi: 10.3389/fimmu.2019.01823. eCollection 2019. Front Immunol. 2019. PMID: 31440237 Free PMC article. Review.
-
Roles of the Glucocorticoid and Mineralocorticoid Receptors in Skin Pathophysiology.Int J Mol Sci. 2018 Jun 29;19(7):1906. doi: 10.3390/ijms19071906. Int J Mol Sci. 2018. PMID: 29966221 Free PMC article. Review.
Cited by
-
Genetic in situ engineering of myeloid regulatory cells controls inflammation in autoimmunity.J Control Release. 2021 Nov 10;339:553-561. doi: 10.1016/j.jconrel.2021.08.040. Epub 2021 Aug 24. J Control Release. 2021. PMID: 34437913 Free PMC article.
-
Central Role of β-1,4-GalT-V in Cancer Signaling, Inflammation, and Other Disease-Centric Pathways.Int J Mol Sci. 2023 Dec 29;25(1):483. doi: 10.3390/ijms25010483. Int J Mol Sci. 2023. PMID: 38203654 Free PMC article. Review.
-
A complete sojourn on nanotechnological advancements and nanocarrier applications in psoriasis management.Naunyn Schmiedebergs Arch Pharmacol. 2025 Jun;398(6):6453-6471. doi: 10.1007/s00210-025-03804-w. Epub 2025 Jan 23. Naunyn Schmiedebergs Arch Pharmacol. 2025. PMID: 39847054 Review.
-
Glucocorticoid-Induced Leucine Zipper-Mediated TLR2 Downregulation Accounts for Reduced Neutrophil Activity Following Acute DEX Treatment.Cells. 2021 Aug 28;10(9):2228. doi: 10.3390/cells10092228. Cells. 2021. PMID: 34571877 Free PMC article.
-
Research progress and perspective in metabolism and metabolomics of psoriasis.Chin Med J (Engl). 2020 Nov 20;133(24):2976-2986. doi: 10.1097/CM9.0000000000001242. Chin Med J (Engl). 2020. PMID: 33237698 Free PMC article.
References
-
- Granner DK, Wang J-C, Yamamoto KR. Regulatory actions of glucocorticoid hormones: from organisms to mechanisms. (New York, NY: Springer, 3–31. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

