Adiponectin improves diabetic nephropathy by inhibiting necrotic apoptosis
- PMID: 31572480
- PMCID: PMC6764294
- DOI: 10.5114/aoms.2018.79570
Adiponectin improves diabetic nephropathy by inhibiting necrotic apoptosis
Abstract
Introduction: This study aimed to investigate the effect of adiponectin (Apn) on necrotic apoptosis (Nec) in vitro and in vivo to clarify the possible role of Apn in the pathogenesis of diabetic nephropathy (DN).
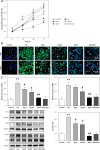
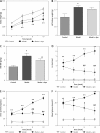
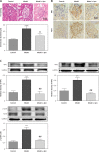
Material and methods: Rat glomerular endothelial (RGE) cells were treated with high glucose (HG, 30 mmol/l) for 24 h and the effects of Apn on cell viability, RIP1 and RIP3 expression and p-p38MAPK activation were assayed by CCK-8, immunofluorescence and western blot. Then a streptozotocin (STZ)-induced DN rat model was established. The body weight, left kidney weight, left kidney weight/body weight (KW/BW), creatinine clearance rate (Ccr), 24 h urine protein and blood glucose were recorded. The expression of RIP1, RIP3 and p-p38MAPK in renal tissues was examined by immunohistochemistry and western blot.
Results: Treatment of RGE cells with HG induced significant cytotoxicity and increased expression levels of RIP1, RIP3 and p-p38MAPK, which were abrogated by Apn in a concentration-dependent manner. In vivo, compared with the control group, the Ccr, 24 h urine protein and the blood glucose level of the rats in the model group were significantly increased, effects which were abrogated by Apn intervention. Moreover, the expression levels of RIP1, PIP3 and p-p38MAPK were also significantly increased in the model group, effects which were canceled by Apn intervention.
Conclusions: Apn can alleviate the inflammatory response and damage of DN by inhibiting Nec via p-p38MAPK signaling.
Keywords: diabetic nephropathy; necroptosis; p38MAPK; rat glomerular endothelial cells.
Copyright: © 2018 Termedia & Banach.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Gantala SR, Kondapalli MS, Kummari R, et al. Collagenase-1 (-1607 1G/2G), gelatinase-A (-1306 C/T), stromelysin-1 (-1171 5A/6A) functional promoter polymorphisms in risk prediction of type 2 diabetic nephropathy. Gene. 2018;673:22–31. - PubMed
-
- Zoja C, Locatelli M, Corna D, et al. Therapy with a selective cannabinoid receptor type 2 agonist limits albuminuria and renal injury in mice with type 2 diabetic nephropathy. Nephron. 2016;132:59–69. - PubMed
-
- Kaifu K, Ueda S, Nakamura N, et al. Advanced glycation end products evoke inflammatory reactions in proximal tubular cells via autocrine production of dipeptidyl peptidase-4. Microvasc Res. 2018;120:90–3. - PubMed
-
- Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–9. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
