Porcine placental extract facilitates memory and learning in aged mice
- PMID: 31572593
- PMCID: PMC6766592
- DOI: 10.1002/fsn3.1156
Porcine placental extract facilitates memory and learning in aged mice
Abstract
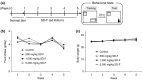
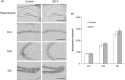
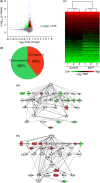
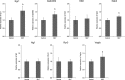
Aging induces a decline in both memory and learning ability without predisposing an individual to diseases of the central nervous system, such as dementia. This decline can have a variety of adverse effects on daily life, and it can also gradually affect the individual and the people they are surrounded by. Since recent evidence indicated that placental extract has effects on brain function such as memory, we hypothesized that placental extract could ameliorate the age-associated reduction in cognitive function in aging. Here, we investigated the effect of new modified porcine placental extract (SD-F) on memory ability in aged mice at both the behavioral and molecular levels. Our results revealed that SD-F significantly enhanced memory ability in the object recognition and object location tasks in a dose-dependent manner in aged mice relative to controls. The numbers of Nissl-positive cells in the hippocampal cornu ammonis 3 (CA3) and dentate gyrus (DG) regions were increased in SD-F-treated aged mice relative to controls. RNA-seq analysis of the hippocampus of aged mice identified 542 differentially expressed genes, of which 216 were up-regulated and 326 were down-regulated in SD-F-treated mice relative to controls. Of the 216 up-regulated genes, we identified four characteristic genes directly related to memory, including early growth response protein 1 (Egr1), growth arrest and DNA-damage-inducible, beta (Gadd45b), NGFI-A binding protein 2 (Nab2), and vascular endothelial growth factor a (Vegfa). These results suggest that the efficacy of SD-F involves upregulation of these genes.
Keywords: RNA‐seq; aging; hippocampus; memory; porcine placental extract.
© 2019 The Authors. Food Science & Nutrition published by Wiley Periodicals, Inc.
Conflict of interest statement
Taiichi Kaku is a stockholder of Japan Bio Products Co., Ltd. Akihiro Yamauchi, Takahiro Tone, Koji Sugimoto, Eiichi Hirano, and Hong Seok Lim are employees of Japan Bio Products Co., Ltd. Chihiro Tohda, Takayuki Shindo, Koji Tamada, and Yoichi Mizukami declare no conflict of interest.
Figures

Similar articles
-
Maintaining Aging Hippocampal Function with Safe and Feasible Shaking Exercise in SAMP10 Mice.Dement Geriatr Cogn Disord. 2020;49(2):185-193. doi: 10.1159/000507884. Epub 2020 Jun 11. Dement Geriatr Cogn Disord. 2020. PMID: 32526748
-
Placental extract improves hippocampal neuronal loss and fear memory impairment resulting from chronic restraint stress in ovariectomized mice.J Pharmacol Sci. 2012;120(2):89-97. doi: 10.1254/jphs.12115fp. Epub 2012 Sep 6. J Pharmacol Sci. 2012. PMID: 22971911 Free PMC article.
-
Dentate Gyrus Sharp Waves, a Local Field Potential Correlate of Learning in the Dentate Gyrus of Mice.J Neurosci. 2020 Sep 9;40(37):7105-7118. doi: 10.1523/JNEUROSCI.2275-19.2020. Epub 2020 Aug 19. J Neurosci. 2020. PMID: 32817247 Free PMC article.
-
Possible involvement of hippocampal immediate-early genes in contextual fear memory deficit induced by cranial irradiation.Neurobiol Learn Mem. 2016 Sep;133:19-29. doi: 10.1016/j.nlm.2016.05.012. Epub 2016 May 30. Neurobiol Learn Mem. 2016. PMID: 27255708
-
The CRISP theory of hippocampal function in episodic memory.Front Neural Circuits. 2013 May 6;7:88. doi: 10.3389/fncir.2013.00088. eCollection 2013. Front Neural Circuits. 2013. PMID: 23653597 Free PMC article. Review.
Cited by
-
Horse Placental Extract Enhances Neurogenesis in the Presence of Amyloid β.Nutrients. 2021 May 14;13(5):1672. doi: 10.3390/nu13051672. Nutrients. 2021. PMID: 34069207 Free PMC article.
-
Equine placental extract supplement as a night barking remedy in dogs with cognitive dysfunction syndrome.Vet Med Sci. 2022 Sep;8(5):1887-1892. doi: 10.1002/vms3.893. Epub 2022 Aug 3. Vet Med Sci. 2022. PMID: 35921448 Free PMC article.
-
Successful treatment of ascites accumulation and diarrhea associated with protein-losing enteropathy with oral equine placenta extract supplementation in a dog: A case report.Open Vet J. 2022 Sep-Oct;12(5):774-781. doi: 10.5455/OVJ.2022.v12.i5.24. Epub 2022 Oct 15. Open Vet J. 2022. PMID: 36589412 Free PMC article.
-
Protective Effect and Mechanism of Placenta Extract on Liver.Nutrients. 2022 Nov 29;14(23):5071. doi: 10.3390/nu14235071. Nutrients. 2022. PMID: 36501102 Free PMC article. Review.
-
The role of Gadd45b in neurologic and neuropsychiatric disorders: An overview.Front Mol Neurosci. 2022 Oct 13;15:1021207. doi: 10.3389/fnmol.2022.1021207. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36311022 Free PMC article. Review.
References
-
- Bak, D. H. , Na, J. , Choi, M. J. , Lee, B. C. , Oh, C. T. , Kim, J. Y. , … Kim, B. J. (2018). Anti‐apoptotic effects of human placental hydrolysate against hepatocyte toxicity in vivo and in vitro. International Journal of Molecular Medicine, 42, 2569–2583. 10.3892/ijmm.2018.3830 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

