Hydrated Sodium Ion Clusters [Na+(H2O)n (n = 1-6)]: An ab initio Study on Structures and Non-covalent Interaction
- PMID: 31572714
- PMCID: PMC6751288
- DOI: 10.3389/fchem.2019.00624
Hydrated Sodium Ion Clusters [Na+(H2O)n (n = 1-6)]: An ab initio Study on Structures and Non-covalent Interaction
Abstract
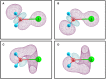
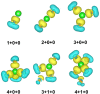
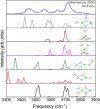
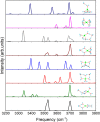
Structural, thermodynamic, and vibrational characteristics of water clusters up to six water molecules incorporating a single sodium ion [Na+(H2O)n (n = 1-6)] are calculated using a comprehensive genetic algorithm combined with density functional theory on global search, followed by high-level ab initio calculation. For n ≥ 4, the coordinated water molecules number for the global minimum of clusters is 4 and the outer water molecules connecting with coordinated water molecules by hydrogen bonds. The charge analysis reveals the electron transfer between sodium ions and water molecules, providing an insight into the variations of properties of O-H bonds in clusters. Moreover, the simulated infrared (IR) spectra with anharmonic correction are in good agreement with the experimental results. The O-H stretching vibration frequencies show redshifts comparing with a free water molecule, which is attributed to the non-covalent interactions, including the ion-water interaction, and hydrogen bonds. Our results exhibit the comprehensive geometries, energies, charge, and anharmonic vibrational properties of Na+(H2O)n (n = 1-6), and reveal a deeper insight of non-covalent interactions.
Keywords: IR spectra; anharmonic effect; hydrated sodium cluster; natural bond orbital; stabilization energy.
Copyright © 2019 Wang, Shi, Su, Tang, Huang and Zhao.
Figures



Similar articles
-
Effects of Na+ and Cl- on hydrated clusters by ab initio study.J Chem Phys. 2023 Jul 28;159(4):044305. doi: 10.1063/5.0159191. J Chem Phys. 2023. PMID: 37489651
-
Structures and Spectroscopic Properties of F-(H2O) n with n = 1-10 Clusters from a Global Search Based On Density Functional Theory.J Phys Chem A. 2018 Apr 5;122(13):3413-3422. doi: 10.1021/acs.jpca.7b08872. Epub 2018 Mar 27. J Phys Chem A. 2018. PMID: 29546760
-
Revisit the landscape of protonated water clusters H+(H2O)n with n = 10-17: An ab initio global search.J Chem Phys. 2018 May 7;148(17):174305. doi: 10.1063/1.5026383. J Chem Phys. 2018. PMID: 29739201
-
A theoretical study on hydrated sodium ion-phenylalanine clusters Na+(Phe)(H2O)n (n = 0-6; Phe = phenylalanine).Phys Chem Chem Phys. 2023 Nov 8;25(43):29576-29584. doi: 10.1039/d3cp03144f. Phys Chem Chem Phys. 2023. PMID: 37877287
-
Exploring the nature of the interactions between the molecules of the sodium dodecyl sulfate and water in crystal phases and in the water/vacuum interface.Heliyon. 2020 Jun 26;6(6):e04199. doi: 10.1016/j.heliyon.2020.e04199. eCollection 2020 Jun. Heliyon. 2020. PMID: 32637679 Free PMC article. Review.
Cited by
-
Supramolecular architecture of sodium heterocyclic dithiocarbamate salt: Insights from spectral analysis and bond valence sum characterization.Heliyon. 2024 Mar 27;10(7):e28642. doi: 10.1016/j.heliyon.2024.e28642. eCollection 2024 Apr 15. Heliyon. 2024. PMID: 38633641 Free PMC article.
-
Modeling Local Aerosol Surface Environments: Clustering of Pyruvic Acid Analogs, Water, and Na+, Cl- Ions.ACS Omega. 2025 Jan 2;10(1):1470-1485. doi: 10.1021/acsomega.4c09196. eCollection 2025 Jan 14. ACS Omega. 2025. PMID: 39829444 Free PMC article.
-
Defining the Collapse Point in Colloidal Unimolecular Polymer (CUP) Formation.Polymers (Basel). 2022 May 7;14(9):1909. doi: 10.3390/polym14091909. Polymers (Basel). 2022. PMID: 35567078 Free PMC article.
-
Ground-State Structures of Hydrated Calcium Ion Clusters From Comprehensive Genetic Algorithm Search.Front Chem. 2021 Jun 30;9:637750. doi: 10.3389/fchem.2021.637750. eCollection 2021. Front Chem. 2021. PMID: 34277560 Free PMC article.
-
Heterogeneous Ion-Induced Nucleation of Water and Butanol Vapors Studied via Computational Quantum Chemistry beyond Prenucleation and Critical Cluster Sizes.J Phys Chem A. 2023 May 11;127(18):3976-3990. doi: 10.1021/acs.jpca.3c00066. Epub 2023 Apr 26. J Phys Chem A. 2023. PMID: 37126596 Free PMC article.
References
-
- Arbman M., Siegbahn H., Pettersson L., Siegbahn P. (1985). Core electron-binding energies and auger-electron energies of solvated clusters - a computational study. Mol. Phys. 54, 1149–1160. 10.1080/00268978500100911 - DOI
-
- Bankura A., Carnevale V., Klein M. L. (2014). Hydration structure of Na+ and K+ fromab initiomolecular dynamics based on modern density functional theory. Mol. Phys. 112, 1448–1456. 10.1080/00268976.2014.905721 - DOI
-
- Barone V., Bloino J., Guido C. A., Lipparini F. (2010). A fully automated implementation of VPT2 Infrared intensities. Chem. Phys. Lett. 496, 157–161. 10.1016/j.cplett.2010.07.012 - DOI
LinkOut - more resources
Full Text Sources

