Bead-Based Multiplex Assay for the Simultaneous Detection of Antibodies to African Swine Fever Virus and Classical Swine Fever Virus
- PMID: 31572739
- PMCID: PMC6753221
- DOI: 10.3389/fvets.2019.00306
Bead-Based Multiplex Assay for the Simultaneous Detection of Antibodies to African Swine Fever Virus and Classical Swine Fever Virus
Abstract
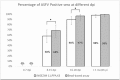
African swine fever (ASF) and Classical swine fever (CSF) are both highly contagious diseases of domestic pigs and wild boar. In the last years, several cases of both diseases have been reported in the Caucasus, Russian Federation and Eastern Europe. Thus, the probability of encountering these two viruses in the same area is increasing. Since differentiation by clinical or post-mortem examination is not possible, laboratory tools for differential diagnosis are required. In the present work, we have developed a triplex bead-based assay using some of the most immunogenic antigens of each virus, for the simultaneous detection of antibodies; i.e. the VP72 and VP30 of ASF virus (ASFV) and the E2 protein of CSF virus (CSFV). The assay was firstly set up and optimized using well characterized reference serum samples specific for each pathogen. Then, a panel of 352 sera from experimentally infected animals with either ASFV or CSFV were analyzed in the multiplex assay. A collection of 253 field negative sera was also included in the study. The results of the multiplex analysis were compared to those obtained by two commercially available ELISAs for detection of antibodies against ASFV or CSFV, and considered in this study as the reference techniques. The data obtained showed values of 97.3% sensitivity and 98.3% specificity for detection of antibodies to ASFV and 95.7% of sensitivity and 99.8% specificity for detection of antibodies to CSFV. This multiplex assay allows the simultaneous and differential detection of antibodies against ASFV and CSFV, providing a valuable tool for surveillance studies. Moreover, this method is rather versatile, offering the possibility of increasing the panel of antigens from other swine diseases that could be of interest for a differential diagnosis along with ASF and CSF.
Keywords: African swine fever; antibody; classical swine fever; diagnosis; multiplex.
Copyright © 2019 Aira, Ruiz, Dixon, Blome, Rueda and Sastre.
Figures
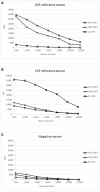
 ) signal of bead #25 coupled to E2, using a reference serum for ASFV (A), a reference serum for CSFV (B) and a negative serum for both diseases (C).
) signal of bead #25 coupled to E2, using a reference serum for ASFV (A), a reference serum for CSFV (B) and a negative serum for both diseases (C).
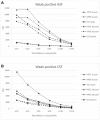
 ) bead #25 coupled to E2. Cut off values for different antigens were established at 3500 (VP72), 3700 (VP30) and 5000 (E2) MFI.
) bead #25 coupled to E2. Cut off values for different antigens were established at 3500 (VP72), 3700 (VP30) and 5000 (E2) MFI.References
-
- Gallardo C, Nieto R, Soler A, Pelayo V, Fernández-Pinero J, Markowska-Daniel I, et al. . Assessment of African swine fever diagnostic techniques as a response to the epidemic outbreaks in Eastern European Union Countries: how to improve surveillance and control programs. J Clin Microbiol. (2015) 53:2555–65. 10.1128/JCM.00857-15 - DOI - PMC - PubMed
-
- Gimenez-Lirola LG, Mur L, Rivera B, Mogler M, Sun Y, Lizano S, et al. . Detection of African swine fever virus antibodies in serum and oral fluid specimens using a recombinant protein 30 (p30) dual matrix indirect ELISA. PLoS ONE. (2016) 11:e0161230. 10.1371/journal.pone.0161230 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

