PhoX: An IMAC-Enrichable Cross-Linking Reagent
- PMID: 31572778
- PMCID: PMC6764163
- DOI: 10.1021/acscentsci.9b00416
PhoX: An IMAC-Enrichable Cross-Linking Reagent
Abstract
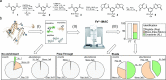
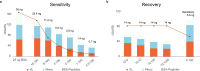
Chemical cross-linking mass spectrometry is rapidly emerging as a prominent technique to study protein structures. Structural information is obtained by covalently connecting peptides in close proximity by small reagents and identifying the resulting peptide pairs by mass spectrometry. However, substoichiometric reaction efficiencies render routine detection of cross-linked peptides problematic. Here, we present a new trifunctional cross-linking reagent, termed PhoX, which is decorated with a stable phosphonic acid handle. This makes the cross-linked peptides amenable to the well-established immobilized metal affinity chromatography (IMAC) enrichment. The handle allows for 300× enrichment efficiency and 97% specificity. We exemplify the approach on various model proteins and protein complexes, e.g., resulting in a structural model of the LRP1/RAP complex. Almost completely removing linear peptides allows PhoX, although noncleavable, to be applied to complex lysates. Focusing the database search to the 1400 most abundant proteins, we were able to identify 1156 cross-links in a single 3 h measurement.
Copyright © 2019 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

