Protective effect of hydrogen sulfide on monocrotaline‑induced pulmonary arterial hypertension via inhibition of the endothelial mesenchymal transition
- PMID: 31573044
- PMCID: PMC6844600
- DOI: 10.3892/ijmm.2019.4359
Protective effect of hydrogen sulfide on monocrotaline‑induced pulmonary arterial hypertension via inhibition of the endothelial mesenchymal transition
Abstract
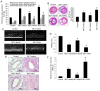
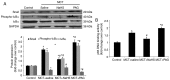
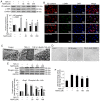
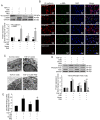
Endothelial‑to‑mesenchymal transition (EndMT) serves an important role in the vascular remodeling of pulmonary arterial hypertension (PAH). However, little is known about the correlation between hydrogen sulfide (H2S), a protective gaseous mediator in PAH and the process of EndMT. Male Sprague‑Dawley rats (10 weeks old) received a single dose of monocrotaline (MCT; i.p., 60 mg/kg) and were randomly treated with NaHS [an H2S donor; intraperitoneal (i.p.) 1 mg/kg/day], DL‑propagylglycine (an inhibitor of H2S synthesis; PAG; i.p., 10 mg/kg/day) or saline, 7 days after MCT injection. Rats were sacrificed 21 days after MCT injection. A selection of human pulmonary artery endothelial cells (HPAECs) were pretreated with NaHS or saline and stimulated with transforming growth factor (TGF)‑β1 (10 ng/ml), and the other HPAECs were transfected with a cystathionine γ‑lyase (CSE, an H2S synthesizing enzyme) plasmid and subsequently stimulated with TGF‑β1. NaHS was indicated to inhibit EndMT and PAH progression by inhibiting the induction of the nuclear factor (NF)‑κB‑Snail pathway. In contrast, the depletion of H2S formation by PAG exacerbated EndMT and PAH by activating NF‑κB‑Snail molecules. In HPAECs, NaHS dose‑dependently inhibited TGF‑β1‑induced EndMT and the activation of the NF‑κB‑Snail pathway. Transfection with a CSE plasmid significantly repressed TGF‑β1‑induced expression of the mesenchymal marker and upregulated the expression of the endothelial marker, which was accompanied by the suppression of the NF‑κB‑Snail pathway. The inhibitory effect of CSE overexpression on TGF‑β1‑induced EndMT was significantly reversed by pretreatment with PAG. In conclusion, the current study provides novel information elucidating the beneficial effect of H2S on PAH through inhibiting the induction of the NF‑κB‑Snail pathway and the subsequent process of EndMT in pulmonary arteries.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

