S‑allyl‑cysteine sulfoxide (alliin) alleviates myocardial infarction by modulating cardiomyocyte necroptosis and autophagy
- PMID: 31573046
- PMCID: PMC6777694
- DOI: 10.3892/ijmm.2019.4351
S‑allyl‑cysteine sulfoxide (alliin) alleviates myocardial infarction by modulating cardiomyocyte necroptosis and autophagy
Abstract
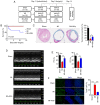
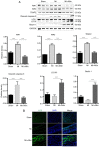
S‑allyl‑cysteine sulfoxide (alliin) is the main organosulfur component of garlic and its preparations. The present study aimed to examine the protective effect of alliin on cardiac function and the underlying mechanism in a mouse model of myocardial infarction (MI). Notably, alliin treatment preserved heart function, attenuated the area of infarction in the myocardium of mice and reduced lesions in the myocardium, including cardiomyocyte fibrosis and death. Further mechanistic experiments revealed that alliin inhibited necroptosis but promoted autophagy in vitro and in vivo. Cell viability assays showed that alliin dose‑dependently reduced the necroptotic index and inhibited the expression of necroptosis‑related receptor‑interacting protein 1, receptor‑interacting protein 3 and tumor necrosis factor receptor‑associated factor 2, whereas the levels of Beclin 1 and microtubule‑associated protein 1 light chain 3, which are associated with autophagy, exhibited an opposite trend upon treatment with alliin. In addition, the level of peroxisome proliferator‑activated receptor γ was increased by alliin. Collectively, these findings demonstrate that alliin has the potential to protect cardiomyocytes from necroptosis following MI and that this protective effect occurs via the enhancement of autophagy.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

