Biological effects of melatonin on human adipose‑derived mesenchymal stem cells
- PMID: 31573052
- PMCID: PMC6844604
- DOI: 10.3892/ijmm.2019.4356
Biological effects of melatonin on human adipose‑derived mesenchymal stem cells
Abstract
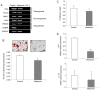
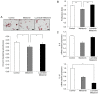
Mesenchymal stem cells (MSCs) are capable of differentiating into other cell types and exhibit immunomodulatory effects. MSCs are affected by several intrinsic and extrinsic signaling modulators, including growth factors, cytokines, extracellular matrix and hormones. Melatonin, produced by the pineal gland, is a hormone that regulates sleep cycles. Recent studies have shown that melatonin improves the therapeutic effects of stem cells. The present study aimed to investigate whether melatonin enhances the biological activities of human adipose‑derived MSCs. The results demonstrated that treatment with melatonin promoted cell proliferation by inducing SRY‑box transcription factor 2 gene expression and preventing replicative senescence. In addition, melatonin exerted anti‑adipogenic effects on MSCs. PCR analysis revealed that the expression of the CCAAT enhancer binding protein a gene, a key transcription factor in adipogenesis, was decreased following melatonin treatment, resulting in reduced adipogenic differentiation in an in vitro assay. The present study also examined the effect of melatonin on the immunomodulatory response using a co‑culture system of human peripheral blood mononuclear cells and MSCs. Activated T cells were strongly inhibited following melatonin exposure compared with those in the control group. Finally, the favorable effects of melatonin on MSCs were confirmed using luzindole, a selective melatonin receptor antagonist. The proliferation‑promoting, anti‑inflammatory effects of melatonin suggested that melatonin‑treated MSCs may be used for effective cell therapy.
Figures



References
-
- Fernández O, Izquierdo G, Fernández V, Leyva L, Reyes V, Guerrero M, León A, Arnaiz C, Navarro G, Páramo MD, et al. Adipose-derived mesenchymal stem cells (AdMSC) for the treatment of secondary-progressive multiple sclerosis: A triple blinded, placebo controlled, randomized phase I/II safety and feasibility study. PLoS One. 2018;13:e0195891. doi: 10.1371/journal.pone.0195891. - DOI - PMC - PubMed

