TRPV1 in experimental autoimmune prostatitis
- PMID: 31573117
- PMCID: PMC7313375
- DOI: 10.1002/pros.23913
TRPV1 in experimental autoimmune prostatitis
Abstract
Background: Chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS) is a disorder that is characterized by persistent pelvic pain in men of any age. Although several studies suggest that the transient receptor potential vanilloid 1 (TRPV1) channel is involved in various pathways of chronic pain, the TRPV1 channel has not been implicated in chronic pelvic pain associated with CP/CPPS.
Methods: Male C57BL/6J (B6) and TRPV1 knockout (TRPV1 KO) mice (5-7 weeks old) were used to study the development of pelvic allodynia in a murine model of CP/CPPS called experimental autoimmune prostatitis (EAP). The prostate lobes, dorsal root ganglia (DRG), and spinal cord were excised at day 20. The prostate lobes were assessed for inflammation, TRPV1 expression, and mast cell activity. DRG and spinal cord, between the L6-S4 regions, were analyzed to determine the levels of phosphorylated ERK1/2 (p-ERK 1/2). To examine the therapeutic potential of TRPV1, B6 mice with EAP received intraurethral infusion of a TRPV1 antagonist at day 20 (repeated every 2 days) and pelvic pain was evaluated at days 20, 25, 30, and 35.
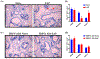
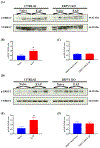
Results: Our data showed that B6 mice with EAP developed pelvic tactile allodynia at days 7, 14, and 20. In contrast, TRPV1 KO mice with EAP do not develop pelvic tactile allodynia at any time point. Although we observed no change in the levels of TRPV1 protein expression in the prostate from B6 mice with EAP, there was evidence of significant inflammation and elevated mast cell activation. Interestingly, the prostate from TRPV1 KO mice with EAP showed a lack of mast cell activation despite evidence of prostate inflammation. Next, we observed a significant increase of p-ERK1/2 in the DRG and spinal cord from B6 mice with EAP; however, p-ERK1/2 expression was unaltered in TRPV1 KO mice with EAP. Finally, we confirmed that intraurethral administration of a TRPV1 antagonist peptide reduced pelvic tactile allodynia in B6 mice with EAP after day 20.
Conclusions: We demonstrated that in a murine model of CP/CPPS, the TRPV1 channel is key to persistent pelvic tactile allodynia and blocking TRPV1 in the prostate may be a promising strategy to quell chronic pelvic pain.
Keywords: CPPS; inflammation; mast cells; pelvic pain; vanilloid 1.
© 2019 Wiley Periodicals, Inc.
Conflict of interest statement
Conflict of interest statement
The authors do not have any conflict of interest to report with regard to the study reported in this manuscript.
Figures


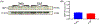

References
-
- Schaeffer AJ, Datta NS, Fowler JE Jr., et al. Overview summary statement. Diagnosis and management of chronic prostatitis/chronic pelvic pain syndrome (CP/CPPS). Urology. 2002;60(6 Suppl):1–4. - PubMed
-
- Tripp DA, Nickel JC, Shoskes D, Koljuskov A. A 2-year follow-up of quality of life, pain, and psychosocial factors in patients with chronic prostatitis/chronic pelvic pain syndrome and their spouses. World J Urol. 2013;31(4):733–739. - PubMed
-
- Rudick CN, Schaeffer AJ, Thumbikat P. Experimental autoimmune prostatitis induces chronic pelvic pain. Am J Physiol Regul Integr Comp Physiol. 2008;294(4):R1268–1275. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

