Contribution of adipogenesis to healthy adipose tissue expansion in obesity
- PMID: 31573549
- PMCID: PMC6763245
- DOI: 10.1172/JCI129191
Contribution of adipogenesis to healthy adipose tissue expansion in obesity
Abstract
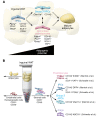
The manner in which white adipose tissue (WAT) expands and remodels directly impacts the risk of developing metabolic syndrome in obesity. Preferential accumulation of visceral WAT is associated with increased risk for insulin resistance, whereas subcutaneous WAT expansion is protective. Moreover, pathologic WAT remodeling, typically characterized by adipocyte hypertrophy, chronic inflammation, and fibrosis, is associated with insulin resistance. Healthy WAT expansion, observed in the "metabolically healthy" obese, is generally associated with the presence of smaller and more numerous adipocytes, along with lower degrees of inflammation and fibrosis. Here, we highlight recent human and rodent studies that support the notion that the ability to recruit new fat cells through adipogenesis is a critical determinant of healthy adipose tissue distribution and remodeling in obesity. Furthermore, we discuss recent advances in our understanding of the identity of tissue-resident progenitor populations in WAT made possible through single-cell RNA sequencing analysis. A better understanding of adipose stem cell biology and adipogenesis may lead to novel strategies to uncouple obesity from metabolic disease.
Conflict of interest statement
Figures


References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

