Effect of Vitamin C Infusion on Organ Failure and Biomarkers of Inflammation and Vascular Injury in Patients With Sepsis and Severe Acute Respiratory Failure: The CITRIS-ALI Randomized Clinical Trial
- PMID: 31573637
- PMCID: PMC6777268
- DOI: 10.1001/jama.2019.11825
Effect of Vitamin C Infusion on Organ Failure and Biomarkers of Inflammation and Vascular Injury in Patients With Sepsis and Severe Acute Respiratory Failure: The CITRIS-ALI Randomized Clinical Trial
Erratum in
-
Data Errors in Table 1 of Study of Vitamin C in Sepsis.JAMA. 2020 Jan 28;323(4):379. doi: 10.1001/jama.2019.21469. JAMA. 2020. PMID: 31990296 Free PMC article. No abstract available.
Abstract
Importance: Experimental data suggest that intravenous vitamin C may attenuate inflammation and vascular injury associated with sepsis and acute respiratory distress syndrome (ARDS).
Objective: To determine the effect of intravenous vitamin C infusion on organ failure scores and biological markers of inflammation and vascular injury in patients with sepsis and ARDS.
Design, setting, and participants: The CITRIS-ALI trial was a randomized, double-blind, placebo-controlled, multicenter trial conducted in 7 medical intensive care units in the United States, enrolling patients (N = 167) with sepsis and ARDS present for less than 24 hours. The study was conducted from September 2014 to November 2017, and final follow-up was January 2018.
Interventions: Patients were randomly assigned to receive intravenous infusion of vitamin C (50 mg/kg in dextrose 5% in water, n = 84) or placebo (dextrose 5% in water only, n = 83) every 6 hours for 96 hours.
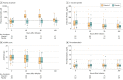
Main outcomes and measures: The primary outcomes were change in organ failure as assessed by a modified Sequential Organ Failure Assessment score (range, 0-20, with higher scores indicating more dysfunction) from baseline to 96 hours, and plasma biomarkers of inflammation (C-reactive protein levels) and vascular injury (thrombomodulin levels) measured at 0, 48, 96, and 168 hours.
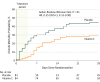
Results: Among 167 randomized patients (mean [SD] age, 54.8 years [16.7]; 90 men [54%]), 103 (62%) completed the study to day 60. There were no significant differences between the vitamin C and placebo groups in the primary end points of change in mean modified Sequential Organ Failure Assessment score from baseline to 96 hours (from 9.8 to 6.8 in the vitamin C group [3 points] and from 10.3 to 6.8 in the placebo group [3.5 points]; difference, -0.10; 95% CI, -1.23 to 1.03; P = .86) or in C-reactive protein levels (54.1 vs 46.1 μg/mL; difference, 7.94 μg/mL; 95% CI, -8.2 to 24.11; P = .33) and thrombomodulin levels (14.5 vs 13.8 ng/mL; difference, 0.69 ng/mL; 95% CI, -2.8 to 4.2; P = .70) at 168 hours.
Conclusions and relevance: In this preliminary study of patients with sepsis and ARDS, a 96-hour infusion of vitamin C compared with placebo did not significantly improve organ dysfunction scores or alter markers of inflammation and vascular injury. Further research is needed to evaluate the potential role of vitamin C for other outcomes in sepsis and ARDS.
Trial registration: ClinicalTrials.gov Identifier: NCT02106975.
Conflict of interest statement
Figures

Comment in
-
Is High-Dose Vitamin C Beneficial for Patients With Sepsis?JAMA. 2019 Oct 1;322(13):1257-1258. doi: 10.1001/jama.2019.11643. JAMA. 2019. PMID: 31573621 No abstract available.
-
[High-dose vitamin C administration in patients with sepsis and acute respiratory distress syndrome: comments on the CITRIS-ALI study].Anaesthesist. 2019 Dec;68(12):852-853. doi: 10.1007/s00101-019-00705-5. Anaesthesist. 2019. PMID: 31754727 German. No abstract available.
-
CITRIS-ALI: How statistics were used to obfuscate the true findings.Anaesth Crit Care Pain Med. 2019 Dec;38(6):575-577. doi: 10.1016/j.accpm.2019.10.004. Anaesth Crit Care Pain Med. 2019. PMID: 31785700 No abstract available.
-
Vitamin C for Sepsis and Acute Respiratory Failure.JAMA. 2020 Feb 25;323(8):792. doi: 10.1001/jama.2019.21981. JAMA. 2020. PMID: 32096841 No abstract available.
-
Vitamin C for Sepsis and Acute Respiratory Failure.JAMA. 2020 Feb 25;323(8):791-792. doi: 10.1001/jama.2019.21984. JAMA. 2020. PMID: 32096842 No abstract available.
References
-
- Roupie E, Lepage E, Wysocki M, et al. ; SRLF Collaborative Group on Mechanical Ventilation; Société de Réanimation de Langue Française . Prevalence, etiologies and outcome of the acute respiratory distress syndrome among hypoxemic ventilated patients. Intensive Care Med. 1999;25(9):920-929. doi: 10.1007/s001340050983 - DOI - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

