Amelioration of metabolic syndrome by metformin associates with reduced indices of low-grade inflammation independently of the gut microbiota
- PMID: 31573841
- PMCID: PMC6962505
- DOI: 10.1152/ajpendo.00245.2019
Amelioration of metabolic syndrome by metformin associates with reduced indices of low-grade inflammation independently of the gut microbiota
Abstract
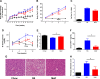
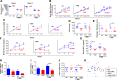
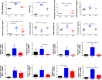
Metformin beneficially impacts several aspects of metabolic syndrome including dysglycemia, obesity, and liver dysfunction, thus making it a widely used frontline treatment for early-stage type 2 diabetes, which is associated with these disorders. Several mechanisms of action for metformin have been proposed, including that it acts as an anti-inflammatory agent, possibly as a result of its impact on intestinal microbiota. In accord with this possibility, we observed herein that, in mice with diet-induced metabolic syndrome, metformin impacts the gut microbiota by preventing its encroachment upon the host, a feature of metabolic syndrome in mice and humans. However, the ability of metformin to beneficially impact metabolic syndrome in mice was not markedly altered by reduction or elimination of gut microbiota, achieved by the use of antibiotics or germfree mice. Although reducing or eliminating microbiota by itself suppressed diet-induced dysglycemia, other features of metabolic syndrome including obesity, hepatic steatosis, and low-grade inflammation remained suppressed by metformin in the presence or absence of gut microbiota. These results support a role for anti-inflammatory activity of metformin, irrespective of gut microbiota, in driving some of the beneficial impacts of this drug on metabolic syndrome.
Keywords: antibiotics; germ free; high-fat diet; metabolic syndrome; metformin; microbiota; steatosis.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures


References
-
- Bailey CJ, Flatt PR, Ewan C. Anorectic effect of metformin in lean and genetically obese hyperglycaemic (ob/ob) mice. Arch Int Pharmacodyn Ther 282: 233–239, 1986. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

