Development of Ovarian Tissue Autograft to Restore Ovarian Function: Protocol for a French Multicenter Cohort Study
- PMID: 31573931
- PMCID: PMC6802486
- DOI: 10.2196/12944
Development of Ovarian Tissue Autograft to Restore Ovarian Function: Protocol for a French Multicenter Cohort Study
Abstract
Background: Sterility is a major late effect of radiotherapy and chemotherapy treatments. Iatrogenic sterility is often permanent and greatly impacts long-term quality of life. Ovarian tissue cryopreservation (OTC) performed before gonadotoxic treatments with subsequent autograft is a method of fertility preservation available for girls and women. Its application in prepubertal girls is of particular value as it is the only possible approach in this patient group. In addition, it does not require a delay in cancer therapy and no ovarian stimulation is needed.
Objective: The primary aim of this protocol is to help increase the implementation of ovarian tissue autografting in France. Knowledge is still lacking regarding the efficacy of ovarian transplantation in restoring ovarian function and regarding the safety of this procedure, especially the risk of cancer cell reseeding in certain types of cancer. A secondary aim of this study is to generate data to improve our understanding of these two essential aspects.
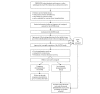
Methods: The DATOR (Development of Ovarian Tissue Autograft in Order to Restore Ovarian Function) study is ongoing in 17 university hospitals. The DATOR protocol includes the autograft of ovarian cortex fragments. Candidates are identified from an observational prospective cohort (called the Prospective Cohort of Patients Candidates for Ovarian Tissue Autograft [PERIDATOR]) of patients who have undergone OTC. Enrollment in the study is initiated at the patient's request and must be validated by the center's multidisciplinary team and by the study steering committee. The DATOR study begins with a total medical checkup. Ovarian tissue qualification and residual disease detection, if required, are performed.
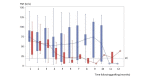
Results: The study is ongoing. Currently, 38 patients have provided informed consent and have been entered into the DATOR study. Graft has been performed for 34 of these patients. An interim analysis was conducted on the first 25 patients for whom the period of at least 1 year posttransplantation was achieved. Out of these 25 patients, 11 women succeeded in becoming pregnant (pregnancy rate=44% [11/25]; delivery rate=40% [10/25]). Among these, 6 women conceived twice, and 1 pregnancy led to a miscarriage.
Conclusions: Our preliminary analysis appears to be coherent with the accumulating body of evidence indicating the potential utility of ovarian tissue autograft for patients with premature ovarian failure. All these elements justify the pursuit of our study.
Trial registration: ClinicalTrials.gov NCT02846064; https://clinicaltrials.gov/ct2/show/NCT02846064.
International registered report identifier (irrid): DERR1-10.2196/12944.
Keywords: cohort study; cryopreservation; fertility preservation; live birth rate; ovarian tissue; pregnancy rate.
©Jean-Baptiste Pretalli, Sophie Frontczak Franck, Lionel Pazart, Christophe Roux, Clotilde Amiot, DATOR Group. Originally published in JMIR Research Protocols (http://www.researchprotocols.org), 30.09.2019.
Conflict of interest statement
Conflicts of Interest: None declared.
Figures

References
-
- Cancer Research UK. [2018-10-24]. Young People's Cancers Statistics http://www.cancerresearchuk.org/health-professional/cancer-statistics/te....
Associated data
LinkOut - more resources
Full Text Sources
Medical

