Oxidative stress mediates ethanol-induced skeletal muscle mitochondrial dysfunction and dysregulated protein synthesis and autophagy
- PMID: 31574345
- PMCID: PMC6910229
- DOI: 10.1016/j.freeradbiomed.2019.09.031
Oxidative stress mediates ethanol-induced skeletal muscle mitochondrial dysfunction and dysregulated protein synthesis and autophagy
Abstract
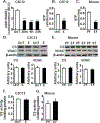
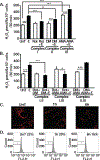
Protein synthesis and autophagy are regulated by cellular ATP content. We tested the hypothesis that mitochondrial dysfunction, including generation of reactive oxygen species (ROS), contributes to impaired protein synthesis and increased proteolysis resulting in tissue atrophy in a comprehensive array of models. In myotubes treated with ethanol, using unbiased approaches, we identified defects in mitochondrial electron transport chain components, endogenous antioxidants, and enzymes regulating the tricarboxylic acid (TCA) cycle. Using high sensitivity respirometry, we observed impaired cellular respiration, decreased function of complexes I, II, and IV, and a reduction in oxidative phosphorylation in ethanol-treated myotubes and muscle from ethanol-fed mice. These perturbations resulted in lower skeletal muscle ATP content and redox ratio (NAD+/NADH). Ethanol also caused a leak of electrons, primarily from complex III, with generation of mitochondrial ROS and reverse electron transport. Oxidant stress with lipid peroxidation (thiobarbituric acid reactive substances) and protein oxidation (carbonylated proteins) were increased in myotubes and skeletal muscle from mice and humans with alcoholic liver disease. Ethanol also impaired succinate oxidation in the TCA cycle with decreased metabolic intermediates. MitoTEMPO, a mitochondrial specific antioxidant, reversed ethanol-induced mitochondrial perturbations (including reduced oxygen consumption, generation of ROS and oxidative stress), increased TCA cycle intermediates, and reversed impaired protein synthesis and the sarcopenic phenotype. We show that ethanol causes skeletal muscle mitochondrial dysfunction, decreased protein synthesis, and increased autophagy, and that these perturbations are reversed by targeting mitochondrial ROS.
Keywords: ATP; Ethanol; Mitochondria; Oxidative stress; Skeletal muscle.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures

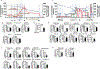




Similar articles
-
Hyperammonaemia-induced skeletal muscle mitochondrial dysfunction results in cataplerosis and oxidative stress.J Physiol. 2016 Dec 15;594(24):7341-7360. doi: 10.1113/JP272796. Epub 2016 Oct 23. J Physiol. 2016. PMID: 27558544 Free PMC article.
-
Reactive oxygen species enhance mitochondrial function, insulin sensitivity and glucose uptake in skeletal muscle of senescence accelerated prone mice SAMP8.Free Radic Biol Med. 2017 Dec;113:267-279. doi: 10.1016/j.freeradbiomed.2017.10.012. Epub 2017 Oct 9. Free Radic Biol Med. 2017. PMID: 29024807
-
Mitochondrial-targeted antioxidants protect skeletal muscle against immobilization-induced muscle atrophy.J Appl Physiol (1985). 2011 Nov;111(5):1459-66. doi: 10.1152/japplphysiol.00591.2011. Epub 2011 Aug 4. J Appl Physiol (1985). 2011. PMID: 21817113 Free PMC article.
-
Redox Control of Proteolysis During Inactivity-Induced Skeletal Muscle Atrophy.Antioxid Redox Signal. 2020 Sep 10;33(8):559-569. doi: 10.1089/ars.2019.8000. Epub 2020 Feb 24. Antioxid Redox Signal. 2020. PMID: 31941357 Free PMC article. Review.
-
Interplay between NADH oxidation by complex I, glutathione redox state and sirtuin-3, and its role in the development of insulin resistance.Biochim Biophys Acta Mol Basis Dis. 2020 Aug 1;1866(8):165801. doi: 10.1016/j.bbadis.2020.165801. Epub 2020 Apr 16. Biochim Biophys Acta Mol Basis Dis. 2020. PMID: 32305451 Review.
Cited by
-
Sarcopenia and frailty in decompensated cirrhosis.J Hepatol. 2021 Jul;75 Suppl 1(Suppl 1):S147-S162. doi: 10.1016/j.jhep.2021.01.025. J Hepatol. 2021. PMID: 34039486 Free PMC article. Review.
-
Alcohol Impairs Bioenergetics and Differentiation Capacity of Myoblasts from Simian Immunodeficiency Virus-Infected Female Macaques.Int J Mol Sci. 2024 Feb 19;25(4):2448. doi: 10.3390/ijms25042448. Int J Mol Sci. 2024. PMID: 38397125 Free PMC article.
-
Alcohol Use Disorders and Their Harmful Effects on the Contractility of Skeletal, Cardiac and Smooth Muscles.Adv Drug Alcohol Res. 2021 Oct;1:10011. doi: 10.3389/ADAR.2021.10011. Epub 2021 Oct 14. Adv Drug Alcohol Res. 2021. PMID: 35169771 Free PMC article.
-
Acute binge alcohol alters whole body metabolism and the time-dependent expression of skeletal muscle-specific metabolic markers for multiple days in mice.Am J Physiol Endocrinol Metab. 2022 Sep 1;323(3):E215-E230. doi: 10.1152/ajpendo.00026.2022. Epub 2022 Jul 6. Am J Physiol Endocrinol Metab. 2022. PMID: 35793479 Free PMC article.
-
Binge drinking leads to an oxidative and metabolic imbalance in skeletal muscle during adolescence in rats: endocrine repercussion.J Physiol Biochem. 2023 Nov;79(4):799-810. doi: 10.1007/s13105-023-00983-z. Epub 2023 Sep 7. J Physiol Biochem. 2023. PMID: 37676577 Free PMC article.
References
-
- Chen Q, Vazquez EJ, Moghaddas S, Hoppel CL, Lesnefsky EJ, Production of reactive oxygen species by mitochondria: central role of complex III, J Biol Chem 278(38) (2003) 36027–31. - PubMed
-
- Ye F, Hoppel CL, Measuring oxidative phosphorylation in human skin fibroblasts, Anal Biochem 437(1) (2013) 52–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

