Insight into the mechanism of ferroptosis inhibition by ferrostatin-1
- PMID: 31574461
- PMCID: PMC6812032
- DOI: 10.1016/j.redox.2019.101328
Insight into the mechanism of ferroptosis inhibition by ferrostatin-1
Abstract
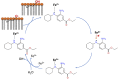
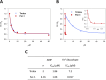
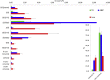
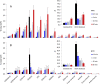
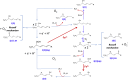
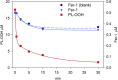
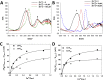
Ferroptosis is a form of cell death primed by iron and lipid hydroperoxides and prevented by GPx4. Ferrostatin-1 (fer-1) inhibits ferroptosis much more efficiently than phenolic antioxidants. Previous studies on the antioxidant efficiency of fer-1 adopted kinetic tests where a diazo compound generates the hydroperoxyl radical scavenged by the antioxidant. However, this reaction, accounting for a chain breaking effect, is only minimally useful for the description of the inhibition of ferrous iron and lipid hydroperoxide dependent peroxidation. Scavenging lipid hydroperoxyl radicals, indeed, generates lipid hydroperoxides from which ferrous iron initiates a new peroxidative chain reaction. We show that when fer-1 inhibits peroxidation, initiated by iron and traces of lipid hydroperoxides in liposomes, the pattern of oxidized species produced from traces of pre-existing hydroperoxides is practically identical to that observed following exhaustive peroxidation in the absence of the antioxidant. This supported the notion that the anti-ferroptotic activity of fer-1 is actually due to the scavenging of initiating alkoxyl radicals produced, together with other rearrangement products, by ferrous iron from lipid hydroperoxides. Notably, fer-1 is not consumed while inhibiting iron dependent lipid peroxidation. The emerging concept is that it is ferrous iron itself that reduces fer-1 radical. This was supported by electroanalytical evidence that fer-1 forms a complex with iron and further confirmed in cells by fluorescence of calcein, indicating a decrease of labile iron in the presence of fer-1. The notion of such as pseudo-catalytic cycle of the ferrostatin-iron complex was also investigated by means of quantum mechanics calculations, which confirmed the reduction of an alkoxyl radical model by fer-1 and the reduction of fer-1 radical by ferrous iron. In summary, GPx4 and fer-1 in the presence of ferrous iron, produces, by distinct mechanism, the most relevant anti-ferroptotic effect, i.e the disappearance of initiating lipid hydroperoxides.
Keywords: Alkoxyl radical; Antioxidant; Ferroptosis; Ferrostatin-1; GPx4; Lipid peroxidation.
Copyright © 2019 The Authors. Published by Elsevier B.V. All rights reserved.
Figures



References
-
- Guiney S.J., Adlard P.A., Bush A.I., Finkelstein D.I., Ayton S. Ferroptosis and cell death mechanisms in Parkinson's disease. Neurochem. Int. 2017;104:34–48. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

