A case report of glecaprevir/pibrentasvir-induced severe hyperbilirubinemia in a patient with compensated liver cirrhosis
- PMID: 31574875
- PMCID: PMC6775421
- DOI: 10.1097/MD.0000000000017343
A case report of glecaprevir/pibrentasvir-induced severe hyperbilirubinemia in a patient with compensated liver cirrhosis
Abstract
Rationale: Glecaprevir/pibrentasvir, a pan-genotypic and ribavirin-free direct acting antiviral agent regimen, has shown significant efficacy and very few serious complications. However, as the drug metabolizes in the liver, it is not recommended in patients with decompensated liver cirrhosis. Herein, we report the case of a patient with compensated liver cirrhosis who developed severe jaundice after glecaprevir/pibrentasvir medication.
Patient concerns: A 77-year-old man diagnosed with chronic hepatitis C-related compensated liver cirrhosis visited hospital due to severe jaundice after 12 weeks of glecaprevir/pibrentasvir medication.
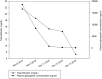
Diagnoses: On the laboratory work-up, the total/direct bilirubin level was markedly elevated to 21.56/11.68 from 1.81 mg/dL; the alanine aminotransferase and aspartate aminotransferase levels were within the normal range. We checked the plasma drug concentration level of glecaprevir, and 18,500 ng/mL was detected, which was more than 15 times higher than the drug concentration level verified in normal healthy adults.
Interventions: Glecaprevir/pibrentasvir was abruptly stopped and after 6 days, the drug concentration level decreased to 35 ng/mL and the serum total/direct bilirubin decreased to 7.49/4.06 mg/dL.
Outcomes: Three months after drug cessation, the serum total bilirubin level normalized to 1.21 mg/dL and HCV RNA was not detected.
Lessons: We report what is likely the first known case of severe jaundice after medication with glecaprevir/pibrentasvir in a patient with compensated liver cirrhosis. Clinicians should bear potential hyperbilirubinemia in mind when treating chronic hepatitis C with this regimen and should monitor the patient closely during follow-up laboratory exams, especially in elderly cirrhotic patients.
Conflict of interest statement
The authors have no conflicts of interest to disclose.
Figures
Similar articles
-
Glecaprevir/pibrentasvir for 8 weeks in treatment-naïve patients with chronic HCV genotypes 1-6 and compensated cirrhosis: The EXPEDITION-8 trial.J Hepatol. 2020 Mar;72(3):441-449. doi: 10.1016/j.jhep.2019.10.020. Epub 2019 Nov 2. J Hepatol. 2020. PMID: 31682879 Clinical Trial.
-
Glecaprevir-pibrentasvir to treat chronic hepatitis C virus infection in Asia: two multicentre, phase 3 studies- a randomised, double-blind study (VOYAGE-1) and an open-label, single-arm study (VOYAGE-2).Lancet Gastroenterol Hepatol. 2020 Sep;5(9):839-849. doi: 10.1016/S2468-1253(20)30086-8. Epub 2020 Jul 16. Lancet Gastroenterol Hepatol. 2020. PMID: 32682494 Clinical Trial.
-
Glecaprevir/pibrentasvir-associated acute liver injury in non-cirrhotic, chronic HCV infection without HBV co-infection.BMJ Case Rep. 2019 May 24;12(5):e226622. doi: 10.1136/bcr-2018-226622. BMJ Case Rep. 2019. PMID: 31129632 Free PMC article.
-
Real-world effectiveness and safety of glecaprevir/pibrentasvir for the treatment of patients with chronic HCV infection: A meta-analysis.J Hepatol. 2020 Jun;72(6):1112-1121. doi: 10.1016/j.jhep.2020.01.025. Epub 2020 Feb 13. J Hepatol. 2020. PMID: 32061651
-
Glecaprevir/Pibrentasvir: The First 8-Week, Pangenotypic HCV Treatment Regimen for Patients 12 Years of Age and Older.Ann Pharmacother. 2020 Mar;54(3):262-276. doi: 10.1177/1060028019877128. Epub 2019 Sep 19. Ann Pharmacother. 2020. PMID: 31537106 Review.
Cited by
-
Severe Liver Injury Associated with Glecaprevir Plus Pibrentasvir Therapy in a Patient with Treatment-naïve Hepatitis C Virus Infection.Intern Med. 2021 Aug 1;60(15):2437-2443. doi: 10.2169/internalmedicine.6664-20. Epub 2021 Feb 22. Intern Med. 2021. PMID: 33612683 Free PMC article.
-
Acute Liver Injury due to Glecaprevir/Pibrentasvir in a Patient with Chronic Hepatitis C Virus Infection without Cirrhosis.Avicenna J Med. 2022 Jul 3;12(3):154-156. doi: 10.1055/s-0042-1750716. eCollection 2022 Jul. Avicenna J Med. 2022. PMID: 36092381 Free PMC article.
-
Glecaprevir-Pibrentasvir and Ethinyl Estradiol-Induced Liver Injury in a Patient Without Cirrhosis.Cureus. 2024 Jun 8;16(6):e61980. doi: 10.7759/cureus.61980. eCollection 2024 Jun. Cureus. 2024. PMID: 38983976 Free PMC article.
-
Indirect hyperbilirubinemia and jaundice during chronic hepatitis C in an HIV-infected patient treated with glecaprevir/pibrentasvir (GLE/PIB) and antiretroviral therapy (ART). The first reported case in Italy.J Prev Med Hyg. 2022 Oct 27;63(3):E420-E423. doi: 10.15167/2421-4248/jpmh2022.63.3.2137. eCollection 2022. J Prev Med Hyg. 2022. PMID: 36415300 Free PMC article.
-
Risk Factors of Glecaprevir/Pibrentasvir-Induced Liver Injury and Efficacy of Ursodeoxycholic Acid.Viruses. 2023 Feb 9;15(2):489. doi: 10.3390/v15020489. Viruses. 2023. PMID: 36851703 Free PMC article.
References
-
- Kanda T. Interferon-free treatment for HCV-infected patients with decompensated cirrhosis. Hepatol Int 2017;11:38–44. - PubMed
-
- European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL Recommendations on Treatment of Hepatitis C 2018. J Hepatol 2018;69:461–511. - PubMed
-
- Kosloski MP, Wang H, Pugatch D, et al. Pharmacokinetics and safety of glecaprevir and pibrentasvir in HCV-negative subjects with hepatic impairment. Eur J Clin Pharmacol 2019;75:217–26. - PubMed
-
- A SPECIAL MEETING REVIEW EDITION: Highlights in Hepatitis C Virus From the 2017 AASLD Liver Meeting: A Review of Selected Presentations From the 2017 AASLD Liver Meeting ∗ October 20-24, 2017 ∗ Washington, DCSpecial Reporting on:∗ Efficacy, Safety, and Pharmacokinetics of Glecaprevir/Pibrentasvir in Adults With Chronic Genotype 1-6 Hepatitis C Virus Infection and Compensated Cirrhosis: An Integrated Analysis∗ Hepatitis C Virus Reinfection and Injecting Risk Behavior Following Elbasvir/Grazoprevir Treatment in Participants on Opiate Agonist Therapy: Co-STAR Part B∗ Efficacy and Safety of Glecaprevir/Pibrentasvir for 8 or 12 Weeks in Treatment-Naive Patients With Chronic HCV Genotype 3: An Integrated Phase 2/3 Analysis∗ SOF/VEL/VOX for 12 Weeks in NS5A-Inhibitor-Experienced HCV-Infected Patients: Results of the Deferred Treatment Group in the Phase 3 POLARIS-1 Study∗ Adherence to Pangenotypic Glecaprevir/Pibrentasvir Treatment and SVR12 in HCV-Infected Patients: An Integrated Analysis of the Phase 2/3 Clinical Trial Program∗ The C-BREEZE 1 and 2 Studies: Efficacy and Safety of Ruzasvir Plus Uprifosbuvir for 12 Weeks in Adults With Chronic Hepatitis C Virus Genotype 1, 2, 3, 4, or 6 Infection100% SVR With 8 Weeks of Ledipasvir/Sofosbuvir in HIV-Infected Men With Acute HCV Infection: Results From the SWIFT-C Trial (Sofosbuvir-Containing Regimens Without Interferon for Treatment of Acute HCV in HIV-1-Infected Individuals)PLUS Meeting Abstract SummariesWith Expert Commentary by:Fred Poordad, MDChief, HepatologyUniversity Transplant CenterClinical Professor of MedicineThe University of Texas Health, San AntonioSan Antonio, Texas. Gastroenterology & hepatology. 2017; 13(12 Suppl 5):1-24. PubMed PMID: 29950953; PubMed Central PMCID: PMC6015237. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical