The efficacy and safety of polydeoxyribonucleotide for the treatment of knee osteoarthritis: Systematic review and meta-analysis of randomized controlled trials
- PMID: 31574892
- PMCID: PMC6775356
- DOI: 10.1097/MD.0000000000017386
The efficacy and safety of polydeoxyribonucleotide for the treatment of knee osteoarthritis: Systematic review and meta-analysis of randomized controlled trials
Abstract
Introduction: The purpose of this study was to use meta-analysis techniques to evaluate the efficacy and safety of polydeoxyribonucleotide (PDRN) injections for knee osteoarthritis (OA) treatment.
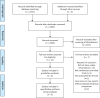
Methods: Multiple comprehensive databases, including MEDLINE, EMBASE, and the Cochrane Library, were searched in November 2018 for studies that compared the effectiveness and safety of intra-articular PDRN injection for the knee joint with hyaluronic acid (HA) injection. Two reviewers independently determined study inclusion and they extracted data using a standardized data extraction form. The predefined primary outcome was Visual Analogue Scale. Secondary outcomes included Knee Injury and Osteoarthritis Outcome Score (KOOS), Knee Society Score (KSS), and adverse events.
Results: Five randomized controlled trials were included in the meta-analysis. After 1 and 2 months, patients in the PDRN group showed significantly better improvement in pain than the HA group (P = .04 and P = .02, respectively). There was no significant difference in pain after 4 months. The pooled analysis showed that no significant differences were seen in function (KOOS and KSS) scores between the PDRN and HA groups (all P > .05) at all time points. There was no significant difference in adverse events between 2 groups (relative risks = 2.15, 95% confidential interval: 0.17-26.67, P = .55).
Conclusion: The intra-articular use of PDRN was similar in function to HA, and the pain-relief effect was superior to HA for 2 months post-injection. Therefore, it could be a favorable alternative to HA to treat persistent pain associated with knee OA while avoiding side effects.Level of evidence I.
Conflict of interest statement
The authors report no conflicts of interest.
Figures


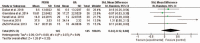

References
-
- Cross M, Smith E, Hoy D, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis 2014;73:1323–30. - PubMed
-
- Johnson VL, Hunter DJ. The epidemiology of osteoarthritis. Best Pract Res Clin Rheumatol 2014;28:5–15. - PubMed
-
- Quintana JM, Arostegui I, Escobar A, et al. Prevalence of knee and hip osteoarthritis and the appropriateness of joint replacement in an older population. Arch Intern Med 2008;168:1576–84. - PubMed
-
- Bellamy N, Campbell J, Robinson V, et al. Intraarticular corticosteroid for treatment of osteoarthritis of the knee. Cochrane Database Syst Rev 2006;Cd005328. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

