Bacopasides I and II Act in Synergy to Inhibit the Growth, Migration and Invasion of Breast Cancer Cell Lines
- PMID: 31574930
- PMCID: PMC6803832
- DOI: 10.3390/molecules24193539
Bacopasides I and II Act in Synergy to Inhibit the Growth, Migration and Invasion of Breast Cancer Cell Lines
Abstract
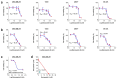
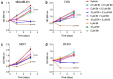
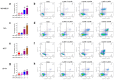
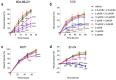
Bacopaside (bac) I and II are triterpene saponins purified from the medicinal herb Bacopa monnieri. Previously, we showed that bac II reduced endothelial cell migration and tube formation and induced apoptosis in colorectal cancer cell lines. The aim of the current study was to examine the effects of treatment with combined doses of bac I and bac II using four cell lines representative of the breast cancer subtypes: triple negative (MDA-MB-231), estrogen receptor positive (T47D and MCF7) and human epidermal growth factor receptor 2 (HER2) positive (BT-474). Drug treatment outcome measures included cell viability, proliferation, cell cycle, apoptosis, migration, and invasion assays. Relationships were analysed by one- and two-way analysis of variance with Bonferroni post-hoc analysis. Combined doses of bac I and bac II, each below their half maximal inhibitory concentration (IC50), were synergistic and reduced the viability and proliferation of the four breast cancer cell lines. Cell loss occurred at the highest dose combinations and was associated with G2/M arrest and apoptosis. Migration in the scratch wound assay was significantly reduced at apoptosis-inducing combinations, but also at non-cytotoxic combinations, for MDA-MB-231 and T47D (p < 0.0001) and BT-474 (p = 0.0003). Non-cytotoxic combinations also significantly reduced spheroid invasion of MDA-MB-231 cells by up to 97% (p < 0.0001). Combining bac I and II below their IC50 reduced the viability, proliferation, and migration and invasiveness of breast cancer cell lines, suggesting synergy between bac I and II.
Keywords: bacopaside I; bacopaside II; breast cancer; migration; spheroid invasion; synergy; triple negative breast cancer.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

