Partially Hydrolyzed Guar Gum Attenuates d-Galactose-Induced Oxidative Stress and Restores Gut Microbiota in Rats
- PMID: 31574948
- PMCID: PMC6801633
- DOI: 10.3390/ijms20194861
Partially Hydrolyzed Guar Gum Attenuates d-Galactose-Induced Oxidative Stress and Restores Gut Microbiota in Rats
Abstract
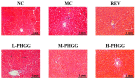
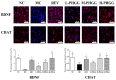
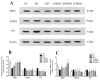
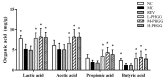
Partially hydrolyzed guar gum (PHGG) has received considerable attention for its various bioactive functions. The injection of d-galactose can cause aging-related injury which is usually resulted from oxidative stress on tissues and cells. In this study, d-galactose (200 mg/kg/day) was injected into rats, and the protective effects of PHGG (500, 1000, and 1500 mg/kg/day) against oxidative damages, as well as its probiotic functions, were analyzed. The results showed that PHGG treatment at a concentration of 1500 mg/kg/day greatly reduced the levels of lactic acid, nitric oxide, inducible nitric oxide synthase, advanced glycation end products, and increased the telomerase activity, by 7.60%, 9.25%, 12.28%, 14.58%, and 9.01%, respectively. Moreover, PHGG significantly elevated the activities of antioxidant enzymes and decreased the content of malondialdehyde in rat serum and brain. The oxidative damage was also significantly alleviated in the liver and hippocampus and the expressions of brain-derived neurotrophic factor and choline acetyltransferase also increased. Furthermore, PHGG treatment could significantly regulated the expression of sirtuin 1, forkhead box O1, and tumor protein p53 in the hippocampus. It also increased the levels of organic acids and improved the composition of intestinal microbiota. These findings demonstrated that PHGG treatment could effectively alleviate the oxidative damage and dysbacteriosis.
Keywords: aging; d-galactose; dysbacteriosis; oxidative damage; partially hydrolyzed guar gum.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures

References
-
- Sanada Y., Asai S., Ikemoto A., Moriwaki T., Nakamura N., Miyaji M., Zhang-Akiyama Q.M. Oxidation resistance 1 is essential for protection against oxidative stress and participates in the regulation of aging in Caenorhabditis elegans. Free Radic. Res. 2014;48:919–928. doi: 10.3109/10715762.2014.927063. - DOI - PubMed
-
- Garcia-Mesa Y., Colie S., Corpas R., Cristofol R., Comellas F., Nebreda A.R., Gimenez-Llort L., Sanfeliu C. Oxidative stress is a central target for physical exercise neuroprotection against pathological brain aging. J. Gerontol. A Biol. Sci. Med. Sci. 2016;71:40–49. doi: 10.1093/gerona/glv005. - DOI - PubMed
-
- Donato A.J., Eskurza I., Silver A.E., Levy A.S., Pierce G.L., Gates P.E., Seals D.R. Direct evidence of endothelial oxidative stress with aging in humans. Relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-κB. Circ. Res. 2007;100:1659–1666. doi: 10.1161/01.RES.0000269183.13937.e8. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

