Baseline Serum Vitamin A and D Levels Determine Benefit of Oral Vitamin A&D Supplements to Humoral Immune Responses Following Pediatric Influenza Vaccination
- PMID: 31575021
- PMCID: PMC6832482
- DOI: 10.3390/v11100907
Baseline Serum Vitamin A and D Levels Determine Benefit of Oral Vitamin A&D Supplements to Humoral Immune Responses Following Pediatric Influenza Vaccination
Abstract
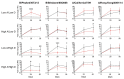
Maximizing vaccine efficacy is critical, but previous research has failed to provide a one-size-fits-all solution. Although vitamin A and vitamin D supplementation studies have been designed to improve vaccine efficacy, experimental results have been inconclusive. Information is urgently needed to explain study discrepancies and to provide guidance for the future use of vitamin supplements at the time of vaccination. We conducted a randomized, blinded, placebo-controlled study of influenza virus vaccination and vitamin supplementation among 2 to 8 (inclusive) year old children over three seasons, including 2015-2016 (n = 9), 2016-2017 (n = 44), and 2017-2018 (n = 26). Baseline measurements of vitamins A and D were obtained from all participants. Measurements were of serum retinol, retinol-binding protein (RBP, a surrogate for retinol), and 25-hydroxyvitamin D (25(OH)D). Participants were stratified into two groups based on high and low incoming levels of RBP. Children received two doses of the seasonal influenza virus vaccine on days 0 and 28, either with an oral vitamin supplement (termed A&D; 20,000 IU retinyl palmitate and 2000 IU cholecalciferol) or a matched placebo. Hemagglutination inhibition (HAI) antibody responses were evaluated toward all four components of the influenza virus vaccines on days 0, 28, and 56. Our primary data were from season 2016-2017, as enrollment was highest in this season and all children exhibited homogeneous and negative HAI responses toward the Phuket vaccine at study entry. Responses among children who entered the study with insufficient or deficient levels of RBP and 25(OH)D benefited from the A&D supplement (p < 0.001 for the day 28 Phuket response), whereas responses among children with replete levels of RBP and 25(OH)D at baseline were unaffected or weakened (p = 0.02 for the day 28 Phuket response). High baseline RBP levels associated with high HAI titers, particularly for children in the placebo group (baseline RBP correlated positively with Phuket HAI titers on day 28, r = 0.6, p = 0.003). In contrast, high baseline 25(OH)D levels associated with weak HAI titers, particularly for children in the A&D group (baseline 25(OH)D correlated negatively with Phuket HAI titers on day 28, r = -0.5, p = 0.02). Overall, our study demonstrates that vitamin A&D supplementation can improve immune responses to vaccines when children are vitamin A and D-insufficient at baseline. Results provide guidance for the appropriate use of vitamins A and D in future clinical vaccine studies.
Keywords: antibody response; baseline; influenza virus vaccine; pediatric; supplement; vitamins A and D.
Conflict of interest statement
J.L.H. reports authorship on a patent for Sendai virus-based vaccine development.
Figures

References
-
- Surman S.L., Jones B.G., Rudraraju R., Sealy R.E., Hurwitz J.L. Intranasal administration of retinyl palmitate with a respiratory virus vaccine corrects impaired mucosal IgA response in the vitamin A-deficient host. Clin. Vaccine Immunol. 2014;21:598–601. doi: 10.1128/CVI.00757-13. - DOI - PMC - PubMed
-
- Martineau A.R., Jolliffe D.A., Hooper R.L., Greenberg L., Aloia J.F., Bergman P., Dubnov-Raz G., Esposito S., Ganmaa D., Ginde A.A., et al. Vitamin D supplementation to prevent acute respiratory tract infections: Systematic review and meta-analysis of individual participant data. BMJ. 2017;356:i6583. doi: 10.1136/bmj.i6583. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

