The NoHoW protocol: a multicentre 2×2 factorial randomised controlled trial investigating an evidence-based digital toolkit for weight loss maintenance in European adults
- PMID: 31575569
- PMCID: PMC6773359
- DOI: 10.1136/bmjopen-2019-029425
The NoHoW protocol: a multicentre 2×2 factorial randomised controlled trial investigating an evidence-based digital toolkit for weight loss maintenance in European adults
Abstract
Introduction: Obesity and associated diseases place a severe burden on healthcare systems. Behavioural interventions for weight loss (WL) are successful in the short term but often result in weight regain over time. Self-regulation of eating and activity behaviours may significantly enhance weight loss maintenance (WLM) and may be effectively augmented by contextual behavioural approaches to emotion regulation. The NoHoW trial tests the efficacy of a theoretically informed, evidence-based digital toolkit using a mobile-enabled website, activity trackers and Wi-Fi scales for WLM aiming to target (1) self-regulation and motivation, and (2) emotion regulation in adults who achieved clinically significant (≥5%) WL in the previous 12 months (initial body mass index (BMI) ≥25 kg/m2).
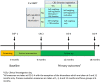
Methods and analysis: The study is an 18-month, 3-centre, 2×2 factorial single-blind, randomised controlled trial, which recruited 1627 participants achieving ≥5% WL between March 2017 and March 2018. Participants are randomly allocated to one of four arms: (1) self-monitoring only (self-weighing and activity tracker), (2) self-regulation and motivation, (3) emotion regulation or (4) combined self-regulation, motivation and emotion regulation. Participants attend four clinical investigation days at 0, 6, 12 and 18 months and are instructed to use the digital toolkit for 18 weeks during the first 6 months and at their discretion for the remaining 12 months. The primary outcome is change in weight (kg) at 12 months from baseline. Secondary outcomes are body composition (eg, bioimpedance analysis), health biomarkers (glycated haemoglobin, lipids, blood pressure, hair cortisol), dietary intake, physical activity, sleep, motivational, self-regulatory, emotion regulatory moderators/mediators of WLM, engagement, user experience, acceptability and cost-effectiveness of the interventions.
Ethics and dissemination: Ethical approval was granted by Institutional Ethics Committees at the Universities of Leeds (17-0082; 27 February 2017), Lisbon (17/2016; 20 February 2017) and Capital Region of Denmark (H-16030495, 8 March 2017). Results will be published in scientific journals.
Trial registration number: ISRCTN88405328.
Keywords: Behaviour change; Digital health; Emotion regulation; Motivation; Self-regulation; Weight loss maintenance.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: RJS consults for Slimming World through the University of Leeds. MMM and GH also conducted consultancy work with Slimming World. Slimming World were a partner of the project to aid recruitment within the UK but will not be involved in the analysis and interpretation of the trial.
Figures
References
-
- Hunt A, Ferguson J. Health costs in the European Union: how much is related to EDCS. Brussels, the health and environmental alliance, 2014. Available: https://www.env-health.org/IMG/pdf/18062014_final_health_costs_in_the_eu... [Accessed 04 Jan 2019].
-
- World Health Organization The challenge of obesity in the who European region and the strategies for response. Copenhagen, Denmark Europe, 2007. Available: http://www.euro.who.int/__data/assets/pdf_file/0008/98243/E89858.pdf [Accessed 04 Jan 2019].