Neurodevelopmental disorders among term infants exposed to antenatal corticosteroids during pregnancy: a population-based study
- PMID: 31575578
- PMCID: PMC6773295
- DOI: 10.1136/bmjopen-2019-031197
Neurodevelopmental disorders among term infants exposed to antenatal corticosteroids during pregnancy: a population-based study
Abstract
Objective: An increasing proportion of fetuses are exposed to antenatal corticosteroids (ACS). Despite their immediate beneficial effects, the long-term safety of ACS has been an ongoing source of concern. In the current study, we assessed the likelihood of neurodevelopmental problems among term infants exposed to ACS earlier in pregnancy compared with non-exposed term infants.
Design: Retrospective cohort study (2006-2011). Median duration of follow-up was 7.8 (IQR 6.4-9.2) years.
Setting: Population-based study, Ontario, Canada.
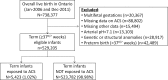
Participants: All live singleton infants born at term (≥370/7 weeks gestation) (n=529 205).
Exposure: ACS during pregnancy.
Primary and secondary outcome measures: A composite of diagnostic or billing codes reflecting proven or suspected neurodevelopmental problems during childhood including audiometry testing, visual testing or physician service claim with a diagnosis code related to a suspected neurocognitive disorder.
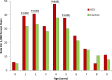
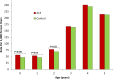
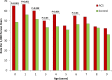
Results: At 5 years of age, the cumulative rate for the primary outcome was higher among infants exposed to ACS compared with non-exposed infants: 61.7% (3346/5423) vs 57.8% (302 520/523 782), respectively (p<0.001; number needed to harm (NNH)=25, 95% CI 19 to 38; adjusted HR (aHR) 1.12, 95% CI 1.08 to 1.16). Similar findings were observed for each of the individual components of the primary outcome: 15.3% vs 12.7% for audiometry testing (p<0.001; NNH=39, 95% CI 29 to 63; aHR 1.18, 95% CI 1.11 to 1.25); 45.4% vs 43.5% for visual testing (p=0.006; NNH=54, 95% CI 31 to 200; aHR 1.08, 95% CI 1.04 to 1.12) and 25.8% vs 21.6% for suspected neurocognitive disorder (p<0.001; NNH=24, 95% CI 19 to 33; aHR 1.16, 95% CI 1.10 to 1.21).
Conclusions: We found an association among term infants between exposure to ACS during pregnancy and healthcare utilisation during childhood related to suspected neurocognitive and neurosensory disorders.
Keywords: antenatal corticosteroids; neurodevelopmental; neurosensory; term.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics 1972;50:515–25. - PubMed
-
- Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH consensus statement 1994;12:1–24. - PubMed
-
- Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. The Cochrane database of systematic reviews 2006. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
