PRC2 functions in development and congenital disorders
- PMID: 31575610
- PMCID: PMC6803372
- DOI: 10.1242/dev.181354
PRC2 functions in development and congenital disorders
Abstract
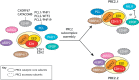
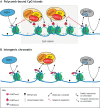
Polycomb repressive complex 2 (PRC2) is a conserved chromatin regulator that is responsible for the methylation of histone H3 lysine 27 (H3K27). PRC2 is essential for normal development and its loss of function thus results in a range of developmental phenotypes. Here, we review the latest advances in our understanding of mammalian PRC2 activity and present an updated summary of the phenotypes associated with its loss of function in mice. We then discuss recent studies that have highlighted regulatory interplay between the modifications laid down by PRC2 and other chromatin modifiers, including NSD1 and DNMT3A. Finally, we propose a model in which the dysregulation of these modifications at intergenic regions is a shared molecular feature of genetically distinct but highly phenotypically similar overgrowth syndromes in humans.
Keywords: DNMT3A; NSD1; PRC2; Sotos syndrome; Tatton-Brown–Rahman syndrome; Weaver syndrome.
© 2019. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures
Similar articles
-
Structural basis for PRC2 engagement with chromatin.Curr Opin Struct Biol. 2021 Apr;67:135-144. doi: 10.1016/j.sbi.2020.10.017. Epub 2020 Nov 21. Curr Opin Struct Biol. 2021. PMID: 33232890 Review.
-
Molecular Mechanisms Directing PRC2 Recruitment and H3K27 Methylation.Mol Cell. 2019 Apr 4;74(1):8-18. doi: 10.1016/j.molcel.2019.03.011. Mol Cell. 2019. PMID: 30951652 Free PMC article. Review.
-
MicroRNA-323-3p regulates the activity of polycomb repressive complex 2 (PRC2) via targeting the mRNA of embryonic ectoderm development (Eed) gene in mouse embryonic stem cells.J Biol Chem. 2013 Aug 16;288(33):23659-65. doi: 10.1074/jbc.M113.475608. Epub 2013 Jul 2. J Biol Chem. 2013. PMID: 23821546 Free PMC article.
-
A Structural Perspective on Gene Repression by Polycomb Repressive Complex 2.Subcell Biochem. 2021;96:519-562. doi: 10.1007/978-3-030-58971-4_17. Subcell Biochem. 2021. PMID: 33252743 Review.
-
Post-translational modifications of PRC2: signals directing its activity.Epigenetics Chromatin. 2020 Oct 31;13(1):47. doi: 10.1186/s13072-020-00369-1. Epigenetics Chromatin. 2020. PMID: 33129354 Free PMC article. Review.
Cited by
-
Know when to fold 'em: Polycomb complexes in oncogenic 3D genome regulation.Front Cell Dev Biol. 2022 Aug 29;10:986319. doi: 10.3389/fcell.2022.986319. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36105358 Free PMC article. Review.
-
De novo purine biosynthesis is a major driver of chemoresistance in glioblastoma.Brain. 2021 May 7;144(4):1230-1246. doi: 10.1093/brain/awab020. Brain. 2021. PMID: 33855339 Free PMC article.
-
Clinical Correlations of Polycomb Repressive Complex 2 in Different Tumor Types.Cancers (Basel). 2021 Jun 24;13(13):3155. doi: 10.3390/cancers13133155. Cancers (Basel). 2021. PMID: 34202528 Free PMC article.
-
A defined glycosylation regulatory network modulates total glycome dynamics during pluripotency state transition.Sci Rep. 2021 Jan 14;11(1):1276. doi: 10.1038/s41598-020-79666-4. Sci Rep. 2021. PMID: 33446700 Free PMC article.
-
The m6A reader YTHDC2 regulates UVB-induced DNA damage repair and histone modification.Photochem Photobiol. 2024 Jul-Aug;100(4):1031-1040. doi: 10.1111/php.13904. Epub 2024 Jan 8. Photochem Photobiol. 2024. PMID: 38190286 Free PMC article.
References
-
- Akasaka T., Kanno M., Balling R., Mieza M. A., Taniguchi M. and Koseki H. (1996). A role for mel-18, a Polycomb group-related vertebrate gene, during the anteroposterior specification of the axial skeleton. Development 122, 1513-1522. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

