Muscle-selective RUNX3 dependence of sensorimotor circuit development
- PMID: 31575648
- PMCID: PMC6826036
- DOI: 10.1242/dev.181750
Muscle-selective RUNX3 dependence of sensorimotor circuit development
Abstract
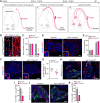
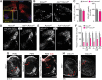
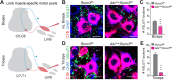
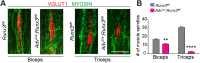
The control of all our motor outputs requires constant monitoring by proprioceptive sensory neurons (PSNs) that convey continuous muscle sensory inputs to the spinal motor network. Yet the molecular programs that control the establishment of this sensorimotor circuit remain largely unknown. The transcription factor RUNX3 is essential for the early steps of PSNs differentiation, making it difficult to study its role during later aspects of PSNs specification. Here, we conditionally inactivate Runx3 in PSNs after peripheral innervation and identify that RUNX3 is necessary for maintenance of cell identity of only a subgroup of PSNs, without discernable cell death. RUNX3 also controls the sensorimotor connection between PSNs and motor neurons at limb level, with muscle-by-muscle variable sensitivities to the loss of Runx3 that correlate with levels of RUNX3 in PSNs. Finally, we find that muscles and neurotrophin 3 signaling are necessary for maintenance of RUNX3 expression in PSNs. Hence, a transcriptional regulator that is crucial for specifying a generic PSN type identity after neurogenesis is later regulated by target muscle-derived signals to contribute to the specialized aspects of the sensorimotor connection selectivity.
Keywords: Dorsal root ganglia; Neuronal specification; Neurotrophins; Sensorimotor circuit; Sensory system.
© 2019. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interestsThe authors declare no competing or financial interests.
Figures
Similar articles
-
Runx3, Brn3a and Isl1 interplay orchestrates the transcriptional program in the early stages of proprioceptive neuron development.PLoS Genet. 2024 Dec 23;20(12):e1011401. doi: 10.1371/journal.pgen.1011401. eCollection 2024 Dec. PLoS Genet. 2024. PMID: 39715266 Free PMC article.
-
Runx3-regulated expression of two Ntrk3 transcript variants in dorsal root ganglion neurons.Dev Neurobiol. 2016 Mar;76(3):313-22. doi: 10.1002/dneu.22316. Epub 2015 Jun 18. Dev Neurobiol. 2016. PMID: 26061886
-
An ensemble of regulatory elements controls Runx3 spatiotemporal expression in subsets of dorsal root ganglia proprioceptive neurons.Genes Dev. 2016 Dec 1;30(23):2607-2622. doi: 10.1101/gad.291484.116. Genes Dev. 2016. PMID: 28007784 Free PMC article.
-
Transcriptional codes and the control of neuronal identity.Annu Rev Neurosci. 2002;25:251-81. doi: 10.1146/annurev.neuro.25.112701.142916. Epub 2002 Mar 27. Annu Rev Neurosci. 2002. PMID: 12052910 Review.
-
Transcriptional networks in the early development of sensory-motor circuits.Curr Top Dev Biol. 2009;87:119-48. doi: 10.1016/S0070-2153(09)01204-6. Curr Top Dev Biol. 2009. PMID: 19427518 Review.
Cited by
-
Whole body vibration training promotes proprioceptive pathway for the treatment of stress urinary incontinence in rats.Transl Androl Urol. 2024 May 31;13(5):657-666. doi: 10.21037/tau-23-675. Epub 2024 May 27. Transl Androl Urol. 2024. PMID: 38855607 Free PMC article.
-
Intrinsic control of neuronal diversity and synaptic specificity in a proprioceptive circuit.Elife. 2020 Aug 18;9:e56374. doi: 10.7554/eLife.56374. Elife. 2020. PMID: 32808924 Free PMC article.
-
Vertebrate Sensory Ganglia: Common and Divergent Features of the Transcriptional Programs Generating Their Functional Specialization.Front Cell Dev Biol. 2020 Oct 26;8:587699. doi: 10.3389/fcell.2020.587699. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33195244 Free PMC article. Review.
-
Touch receptor end-organ innervation and function require sensory neuron expression of the transcription factor Meis2.Elife. 2024 Feb 22;12:RP89287. doi: 10.7554/eLife.89287. Elife. 2024. PMID: 38386003 Free PMC article.
-
Single-cell RNA-sequencing analysis of the developing mouse inner ear identifies molecular logic of auditory neuron diversification.Nat Commun. 2022 Jul 5;13(1):3878. doi: 10.1038/s41467-022-31580-1. Nat Commun. 2022. PMID: 35790771 Free PMC article.
References
-
- Brohmann H., Jagla K. and Birchmeier C. (2000). The role of Lbx1 in migration of muscle precursor cells. Development 127, 437-445. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

