Targeting Histone Chaperone FACT Complex Overcomes 5-Fluorouracil Resistance in Colon Cancer
- PMID: 31575655
- PMCID: PMC6946866
- DOI: 10.1158/1535-7163.MCT-19-0600
Targeting Histone Chaperone FACT Complex Overcomes 5-Fluorouracil Resistance in Colon Cancer
Abstract
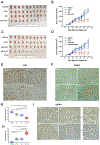
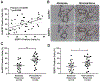
Fluorouracil (5-FU) remains a first-line chemotherapeutic agent for colorectal cancer. However, a subset of colorectal cancer patients who have defective mismatch-repair (dMMR) pathway show resistance to 5-FU. Here, we demonstrate that the efficacy of 5-FU in dMMR colorectal cancer cells is largely dependent on the DNA base excision repair (BER) pathway. Downregulation of APE1, a key enzyme in the BER pathway, decreases IC50 of 5-FU in dMMR colorectal cancer cells by 10-fold. Furthermore, we discover that the facilitates chromatin transcription (FACT) complex facilitates 5-FU repair in DNA via promoting the recruitment and acetylation of APE1 (AcAPE1) to damage sites in chromatin. Downregulation of FACT affects 5-FU damage repair in DNA and sensitizes dMMR colorectal cancer cells to 5-FU. Targeting the FACT complex with curaxins, a class of small molecules, significantly improves the 5-FU efficacy in dMMR colorectal cancer in vitro (∼50-fold decrease in IC50) and in vivo xenograft models. We show that primary tumor tissues of colorectal cancer patients have higher FACT and AcAPE1 levels compared with adjacent nontumor tissues. Additionally, there is a strong clinical correlation of FACT and AcAPE1 levels with colorectal cancer patients' response to chemotherapy. Together, our study demonstrates that targeting FACT with curaxins is a promising strategy to overcome 5-FU resistance in dMMR colorectal cancer patients.
©2019 American Association for Cancer Research.
Conflict of interest statement
Figures
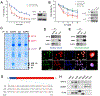
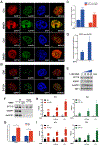
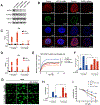
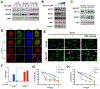

Similar articles
-
Histone chaperone FACT complex inhibitor CBL0137 interferes with DNA damage repair and enhances sensitivity of medulloblastoma to chemotherapy and radiation.Cancer Lett. 2021 Nov 1;520:201-212. doi: 10.1016/j.canlet.2021.07.020. Epub 2021 Jul 14. Cancer Lett. 2021. PMID: 34271103 Free PMC article.
-
Inhibition of the FACT Complex Targets Aberrant Hedgehog Signaling and Overcomes Resistance to Smoothened Antagonists.Cancer Res. 2021 Jun 1;81(11):3105-3120. doi: 10.1158/0008-5472.CAN-20-3186. Epub 2021 Apr 14. Cancer Res. 2021. PMID: 33853831
-
Therapeutic targeting of the MYC signal by inhibition of histone chaperone FACT in neuroblastoma.Sci Transl Med. 2015 Nov 4;7(312):312ra176. doi: 10.1126/scitranslmed.aab1803. Sci Transl Med. 2015. PMID: 26537256 Free PMC article.
-
The FAcilitates Chromatin Transcription (FACT) complex: Its roles in DNA repair and implications for cancer therapy.DNA Repair (Amst). 2022 Jan;109:103246. doi: 10.1016/j.dnarep.2021.103246. Epub 2021 Nov 16. DNA Repair (Amst). 2022. PMID: 34847380 Review.
-
The histone chaperone FACT: a guardian of chromatin structure integrity.Transcription. 2022 Feb-Jun;13(1-3):16-38. doi: 10.1080/21541264.2022.2069995. Epub 2022 Apr 29. Transcription. 2022. PMID: 35485711 Free PMC article. Review.
Cited by
-
Fine-tuning of DNA base excision/strand break repair via acetylation.DNA Repair (Amst). 2020 Sep;93:102931. doi: 10.1016/j.dnarep.2020.102931. DNA Repair (Amst). 2020. PMID: 33087268 Free PMC article. Review.
-
Clinical significance of neoadjuvant chemotherapy for locally advanced colorectal cancer patients with deficient mismatch repair: possibly residual value in the era of immunotherapy.Therap Adv Gastroenterol. 2023 Jan 19;16:17562848221150306. doi: 10.1177/17562848221150306. eCollection 2023. Therap Adv Gastroenterol. 2023. PMID: 36742014 Free PMC article.
-
APE1 and SSRP1 is overexpressed in muscle invasive bladder cancer and associated with poor survival.Heliyon. 2021 Apr 16;7(4):e06756. doi: 10.1016/j.heliyon.2021.e06756. eCollection 2021 Apr. Heliyon. 2021. PMID: 33948507 Free PMC article.
-
Histone Chaperones and Digestive Cancer: A Review of the Literature.Cancers (Basel). 2022 Nov 14;14(22):5584. doi: 10.3390/cancers14225584. Cancers (Basel). 2022. PMID: 36428674 Free PMC article. Review.
-
Revisiting Two Decades of Research Focused on Targeting APE1 for Cancer Therapy: The Pros and Cons.Cells. 2023 Jul 20;12(14):1895. doi: 10.3390/cells12141895. Cells. 2023. PMID: 37508559 Free PMC article. Review.
References
-
- Carethers JM, Smith EJ, Behling CA, Nguyen L, Tajima A, Doctolero RT, et al. Use of 5-fluorouracil and survival in patients with microsatellite-unstable colorectal cancer. Gastroenterology 2004;126(2):394–401. - PubMed
-
- Sinicrope FA, Mahoney MR, Smyrk TC, Thibodeau SN, Warren RS, Bertagnolli MM, et al. Prognostic impact of deficient DNA mismatch repair in patients with stage III colon cancer from a randomized trial of FOLFOX-based adjuvant chemotherapy. J Clin Oncol 2013;31(29):3664–72 doi 10.1200/JCO.2013.48.9591. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

