Telomere repeat-binding factor 2 binds extensively to extra-telomeric G-quadruplexes and regulates the epigenetic status of several gene promoters
- PMID: 31575660
- PMCID: PMC6879327
- DOI: 10.1074/jbc.RA119.008687
Telomere repeat-binding factor 2 binds extensively to extra-telomeric G-quadruplexes and regulates the epigenetic status of several gene promoters
Abstract
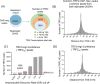
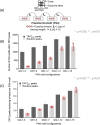
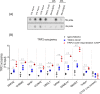
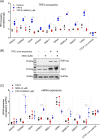
The role of the telomere repeat-binding factor 2 (TRF2) in telomere maintenance is well-established. However, recent findings suggest that TRF2 also functions outside telomeres, but relatively little is known about this function. Herein, using genome-wide ChIP-Seq assays of TRF2-bound chromatin from HT1080 fibrosarcoma cells, we identified thousands of TRF2-binding sites within the extra-telomeric genome. In light of this observation, we asked how TRF2 occupancy is organized within the genome. Interestingly, we found that extra-telomeric TRF2 sites throughout the genome are enriched in potential G-quadruplex-forming DNA sequences. Furthermore, we validated TRF2 occupancy at several promoter G-quadruplex motifs, which did adopt quadruplex forms in solution. TRF2 binding altered expression and the epigenetic state of several target promoters, indicated by histone modifications resulting in transcriptional repression of eight of nine genes investigated here. Furthermore, TRF2 occupancy and target gene expression were also sensitive to the well-known intracellular G-quadruplex-binding ligand 360A. Together, these results reveal an extensive genome-wide association of TRF2 outside telomeres and that it regulates gene expression in a G-quadruplex-dependent fashion.
Keywords: DNA secondary structure; DNA transcription; DNA-binding protein; G-quadruplex; TRF2; epigenetics; extra-telomeric; gene regulation; genome-wide; histone mark; shelterin complex; telomere; telomere repeat-binding factor 2.
© 2019 Mukherjee et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures


Comment in
-
From the wings to the center stage of chromosomes.J Biol Chem. 2019 Nov 22;294(47):17723-17724. doi: 10.1074/jbc.H119.011587. J Biol Chem. 2019. PMID: 31757801 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

