Hydrodynamic Shape Changes Underpin Nuclear Rerouting in Branched Hyphae of an Oomycete Pathogen
- PMID: 31575765
- PMCID: PMC6775453
- DOI: 10.1128/mBio.01516-19
Hydrodynamic Shape Changes Underpin Nuclear Rerouting in Branched Hyphae of an Oomycete Pathogen
Abstract
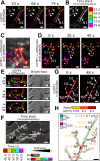
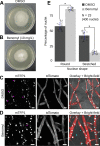
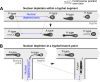
Multinucleate fungi and oomycetes are phylogenetically distant but structurally similar. To address whether they share similar nuclear dynamics, we carried out time-lapse imaging of fluorescently labeled Phytophthora palmivora nuclei. Nuclei underwent coordinated bidirectional movements during plant infection. Within hyphal networks growing in planta or in axenic culture, nuclei either are dragged passively with the cytoplasm or actively become rerouted toward nucleus-depleted hyphal sections and often display a very stretched shape. Benomyl-induced depolymerization of microtubules reduced active movements and the occurrence of stretched nuclei. A centrosome protein localized at the leading end of stretched nuclei, suggesting that, as in fungi, astral microtubule-guided movements contribute to nuclear distribution within oomycete hyphae. The remarkable hydrodynamic shape adaptations of Phytophthora nuclei contrast with those in fungi and likely enable them to migrate over longer distances. Therefore, our work summarizes mechanisms which enable a near-equal nuclear distribution in an oomycete. We provide a basis for computational modeling of hydrodynamic nuclear deformation within branched tubular networks.IMPORTANCE Despite their fungal morphology, oomycetes constitute a distinct group of protists related to brown algae and diatoms. Many oomycetes are pathogens and cause diseases of plants, insects, mammals, and humans. Extensive efforts have been made to understand the molecular basis of oomycete infection, but durable protection against these pathogens is yet to be achieved. We use a plant-pathogenic oomycete to decipher a key physiological aspect of oomycete growth and infection. We show that oomycete nuclei travel actively and over long distances within hyphae and during infection. Such movements require microtubules anchored on the centrosome. Nuclei hydrodynamically adapt their shape to travel in or against the flow. In contrast, fungi lack a centrosome and have much less flexible nuclei. Our findings provide a basis for modeling of flexible nuclear shapes in branched hyphal networks and may help in finding hard-to-evade targets to develop specific antioomycete strategies and achieve durable crop disease protection.
Keywords: Phytophthora palmivora; centrosome; hydrodynamics; nucleus movement; oomycetes.
Copyright © 2019 Evangelisti et al.
Figures





Similar articles
-
Effects of latrunculin B on the actin cytoskeleton and hyphal growth in Phytophthora infestans.Fungal Genet Biol. 2012 Dec;49(12):1014-22. doi: 10.1016/j.fgb.2012.09.008. Epub 2012 Oct 2. Fungal Genet Biol. 2012. PMID: 23036581
-
Cytoplasmic bulk flow propels nuclei in mature hyphae of Neurospora crassa.Eukaryot Cell. 2009 Dec;8(12):1880-90. doi: 10.1128/EC.00062-09. Epub 2009 Aug 14. Eukaryot Cell. 2009. PMID: 19684281 Free PMC article.
-
Microtubules in Candida albicans hyphae drive nuclear dynamics and connect cell cycle progression to morphogenesis.Eukaryot Cell. 2005 Oct;4(10):1697-711. doi: 10.1128/EC.4.10.1697-1711.2005. Eukaryot Cell. 2005. PMID: 16215177 Free PMC article.
-
Phytophthora parasitica: a model oomycete plant pathogen.Mycology. 2014 Jun;5(2):43-51. doi: 10.1080/21501203.2014.917734. Epub 2014 May 19. Mycology. 2014. PMID: 24999436 Free PMC article. Review.
-
Cell biology of plant-oomycete interactions.Cell Microbiol. 2007 Jan;9(1):31-9. doi: 10.1111/j.1462-5822.2006.00833.x. Epub 2006 Nov 1. Cell Microbiol. 2007. PMID: 17081190 Review.
Cited by
-
MycoRed: Betalain pigments enable in vivo real-time visualisation of arbuscular mycorrhizal colonisation.PLoS Biol. 2021 Jul 14;19(7):e3001326. doi: 10.1371/journal.pbio.3001326. eCollection 2021 Jul. PLoS Biol. 2021. PMID: 34260583 Free PMC article.
-
N-acetyltransferase AAC(3)-I confers gentamicin resistance to Phytophthora palmivora and Phytophthora infestans.BMC Microbiol. 2019 Nov 27;19(1):265. doi: 10.1186/s12866-019-1642-0. BMC Microbiol. 2019. PMID: 31775609 Free PMC article.
-
The N-terminal domains of NLR immune receptors exhibit structural and functional similarities across divergent plant lineages.Plant Cell. 2024 Jul 2;36(7):2491-2511. doi: 10.1093/plcell/koae113. Plant Cell. 2024. PMID: 38598645 Free PMC article.
-
Receptor-like cytoplasmic kinases of different subfamilies differentially regulate SOBIR1/BAK1-mediated immune responses in Nicotiana benthamiana.Nat Commun. 2024 May 21;15(1):4339. doi: 10.1038/s41467-024-48313-1. Nat Commun. 2024. PMID: 38773116 Free PMC article.
-
Viable protoplast isolation, organelle visualization and transformation of the globally distributed plant pathogen Phytophthora cinnamomi.Protoplasma. 2024 Sep;261(5):1073-1092. doi: 10.1007/s00709-024-01953-y. Epub 2024 May 4. Protoplasma. 2024. PMID: 38702562 Free PMC article.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

