Increased Epithelial Oxygenation Links Colitis to an Expansion of Tumorigenic Bacteria
- PMID: 31575772
- PMCID: PMC6775460
- DOI: 10.1128/mBio.02244-19
Increased Epithelial Oxygenation Links Colitis to an Expansion of Tumorigenic Bacteria
Abstract
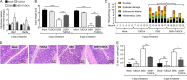
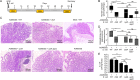
Intestinal inflammation is a risk factor for colorectal cancer formation, but the underlying mechanisms remain unknown. Here, we investigated whether colitis alters the colonic microbiota to enhance its cancer-inducing activity. Colitis increased epithelial oxygenation in the colon of mice and drove an expansion of Escherichia coli within the gut-associated microbial community through aerobic respiration. An aerobic expansion of colibactin-producing E. coli was required for the cancer-inducing activity of this pathobiont in a mouse model of colitis-associated colorectal cancer formation. We conclude that increased epithelial oxygenation in the colon is associated with an expansion of a prooncogenic driver species, thereby increasing the cancer-inducing activity of the microbiota.IMPORTANCE One of the environmental factors important for colorectal cancer formation is the gut microbiota, but the habitat filters that control its cancer-inducing activity remain unknown. Here, we show that chemically induced colitis elevates epithelial oxygenation in the colon, thereby driving an expansion of colibactin-producing Escherichia coli, a prooncogenic driver species. These data suggest that elevated epithelial oxygenation is a potential risk factor for colorectal cancer formation because the consequent changes in the gut habitat escalate the cancer-inducing activity of the microbiota.
Keywords: Escherichia coli; colibactin; colorectal cancer; microbiome.
Copyright © 2019 Cevallos et al.
Figures
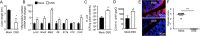

References
-
- Arthur JC, Perez-Chanona E, Muhlbauer M, Tomkovich S, Uronis JM, Fan TJ, Campbell BJ, Abujamel T, Dogan B, Rogers AB, Rhodes JM, Stintzi A, Simpson KW, Hansen JJ, Keku TO, Fodor AA, Jobin C. 2012. Intestinal inflammation targets cancer-inducing activity of the microbiota. Science 338:120–123. doi:10.1126/science.1224820. - DOI - PMC - PubMed
-
- Dejea CM, Fathi P, Craig JM, Boleij A, Taddese R, Geis AL, Wu X, DeStefano Shields CE, Hechenbleikner EM, Huso DL, Anders RA, Giardiello FM, Wick EC, Wang H, Wu S, Pardoll DM, Housseau F, Sears CL. 2018. Patients with familial adenomatous polyposis harbor colonic biofilms containing tumorigenic bacteria. Science 359:592–597. doi:10.1126/science.aah3648. - DOI - PMC - PubMed

