Unraveling FATP1, regulated by ER-β, as a targeted breast cancer innovative therapy
- PMID: 31575907
- PMCID: PMC6773857
- DOI: 10.1038/s41598-019-50531-3
Unraveling FATP1, regulated by ER-β, as a targeted breast cancer innovative therapy
Abstract
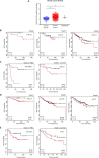
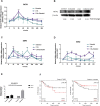
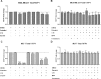
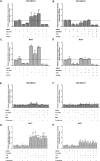
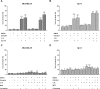
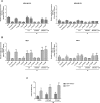
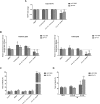
The biochemical demands associated with tumor proliferation prompt neoplastic cells to augment the import of nutrients to sustain their survival and fuel cell growth, with a consequent metabolic remodeling. Fatty acids (FA) are crucial in this process, since they have a dual role as energetic coins and building blocks. Recently, our team has shown that FATP1 has a pivotal role in FA transfer between breast cancer cells (BCCs) and non-cancerous cells in the microenvironment. We aimed to investigate the role of FATP1 in BCCs and also to explore if FATP1 inhibition is a promising therapeutic strategy. In patients' data, we showed a higher expression of FATP1/SLC27A1 in TNBC, which correlated with a significant decreased overall survival (OS). In vitro, we verified that FA and estradiol stimulated FATP1/SLC27A1 expression in BCCs. Additionally, experiments with estradiol and PHTPP (ER-β antagonist) showed that estrogen receptor-β (ER-β) regulates FATP1/SLC27A1 expression, the uptake of FA and cell viability, in four BCC lines. Furthermore, the inhibition of FATP1 with arylpiperazine 5k (DS22420314) interfered with the uptake of FA and cell viability. Our study, unraveled FATP1 as a putative therapeutic target in breast cancer (BC).
Conflict of interest statement
The authors declare no competing interests.
Figures

Similar articles
-
In vivo and in vitro insulin and fasting control of the transmembrane fatty acid transport proteins in Atlantic salmon (Salmo salar).Am J Physiol Regul Integr Comp Physiol. 2011 Oct;301(4):R947-57. doi: 10.1152/ajpregu.00289.2011. Epub 2011 Jul 20. Am J Physiol Regul Integr Comp Physiol. 2011. PMID: 21775646
-
Amyloid beta25-35 impairs docosahexaenoic acid efflux by down-regulating fatty acid transport protein 1 (FATP1/SLC27A1) protein expression in human brain capillary endothelial cells.J Neurochem. 2019 Aug;150(4):385-401. doi: 10.1111/jnc.14722. Epub 2019 Jun 25. J Neurochem. 2019. PMID: 31091338
-
The role of FATP1 in lipid accumulation: a review.Mol Cell Biochem. 2021 Apr;476(4):1897-1903. doi: 10.1007/s11010-021-04057-w. Epub 2021 Jan 24. Mol Cell Biochem. 2021. PMID: 33486652 Review.
-
The blood-brain barrier and protein-mediated fatty acid uptake: role of the blood-brain barrier as a metabolic barrier: An Editorial Comment for 'The blood-brain barrier fatty acid transport protein 1 (FATP1/SLC27A1) supplies docosahexaenoic acid to the brain, and insulin facilitates transport'.J Neurochem. 2017 May;141(3):324-329. doi: 10.1111/jnc.14000. Epub 2017 Mar 30. J Neurochem. 2017. PMID: 28369884
-
Targeting fatty acid synthase in breast and endometrial cancer: An alternative to selective estrogen receptor modulators?Endocrinology. 2006 Sep;147(9):4056-66. doi: 10.1210/en.2006-0486. Epub 2006 Jun 29. Endocrinology. 2006. PMID: 16809439 Review.
Cited by
-
Estrogens and Progestogens in Triple Negative Breast Cancer: Do They Harm?Cancers (Basel). 2021 May 21;13(11):2506. doi: 10.3390/cancers13112506. Cancers (Basel). 2021. PMID: 34063736 Free PMC article. Review.
-
Prostate Cancer Progression: as a Matter of Fats.Front Oncol. 2021 Jul 27;11:719865. doi: 10.3389/fonc.2021.719865. eCollection 2021. Front Oncol. 2021. PMID: 34386430 Free PMC article. Review.
-
Lipid metabolic reprogramming in tumor microenvironment: from mechanisms to therapeutics.J Hematol Oncol. 2023 Sep 12;16(1):103. doi: 10.1186/s13045-023-01498-2. J Hematol Oncol. 2023. PMID: 37700339 Free PMC article. Review.
-
Reduced Expression of Very-Long-Chain Acyl-CoA Synthetases SLC27A4 and SLC27A6 in the Glioblastoma Tumor Compared to the Peritumoral Area.Brain Sci. 2023 May 7;13(5):771. doi: 10.3390/brainsci13050771. Brain Sci. 2023. PMID: 37239243 Free PMC article.
-
Glucagon and Glucose Availability Influence Metabolic Heterogeneity and Malignancy in Pancreatic Neuroendocrine Tumour (pNET) Cells: Novel Routes for Therapeutic Targeting.Molecules. 2025 Jun 25;30(13):2736. doi: 10.3390/molecules30132736. Molecules. 2025. PMID: 40649254 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

