Vitamin D3 regulates apoptosis and proliferation in the testis of D-galactose-induced aged rat model
- PMID: 31575929
- PMCID: PMC6773724
- DOI: 10.1038/s41598-019-50679-y
Vitamin D3 regulates apoptosis and proliferation in the testis of D-galactose-induced aged rat model
Abstract
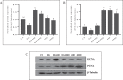
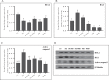
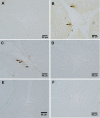
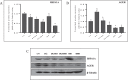
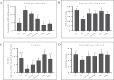
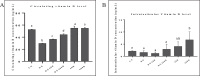
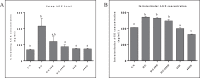
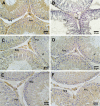
The age-associated imbalances between proliferation and apoptosis lead to impaired spermatogenesis and infertility. The age-associated decline in vitamin D3 levels has been reported and suggested the anti-aging potential of vitamin D3. However, the age-associated decline levels of vitamin D3 has not been studied in relation to the testicular activity. Thus, we investigated the effect of vitamin D3 on the expression of testicular proliferation markers, apoptotic markers, antioxidants system and oxidative stress in a D-gal-induced aged rat model. The present study investigated the levels of vitamin D3 and AGE in serum and testes along with the expression of the AGE-receptor (AGER) in the testis. Vitamin D3 treatment significantly increases cell proliferation and decreases apoptosis in a D-gal-induced aged rat testis. Furthermore, vitamin D3 significantly decreases oxidative stress in aged rat testis by improving the antioxidant defense systems. The expression of AGER was down-regulated by vitamin D3 treatment in aged testis. The circulating and intra-testicular AGE was higher in aged groups, however, only circulating vitamin D3 levels decreased in aged groups. The immunolocalization of VDR showed increased immunostaining in the testis by vitamin D3 treatment. Thus, it can be concluded that vitamin D3 delays testicular senescence by regulating proliferation and apoptosis.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

