Clostridium butyricum alleviates intestinal low-grade inflammation in TNBS-induced irritable bowel syndrome in mice by regulating functional status of lamina propria dendritic cells
- PMID: 31576093
- PMCID: PMC6767978
- DOI: 10.3748/wjg.v25.i36.5469
Clostridium butyricum alleviates intestinal low-grade inflammation in TNBS-induced irritable bowel syndrome in mice by regulating functional status of lamina propria dendritic cells
Abstract
Background: Irritable bowel syndrome (IBS) is one of the most common functional gas-troenterological diseases characterized by abnormal visceral sensitivity and low-grade inflammation. The role of Clostridium butyricum (C. butyricum) in reducing intestinal low-grade inflammation via immune pathways has been well defined. However, the detailed mechanisms of the effects of C. butyricum on intestinal mucosal immunity, especially on immune cells of the lamina propria, remain unclear. Dendritic cells (DCs), which are important immune cells, secrete proinflammatory cytokines (IL-1β, IL-6, and others) and express T cell immuno-globulin and mucin domain-3 (TIM3), promoting proliferation and activation of DCs, and mediating Th1 and Th17 inflammatory responses.
Aim: To investigate the role of DCs in the development of IBS in a rat model and to understand the regulation of DCs after C. butyricum intervention.
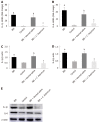
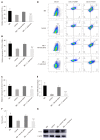
Methods: An IBS animal model was established using C57BL/6 mice, and C. butyricum was continuously administered via the intragastric route to simulate different intestinal immune states. Intestinal visceral hypersensitivity and histopathology were assessed using the abdominal withdrawal reflex (AWR) test and hematoxylin & eosin (H&E) staining, respectively. The expression of proinflammatory cytokines (IL-1β and IL-6) and TIM3 was analyzed by Western blot analysis and real-time PCR. Flow cytometry was applied to analyze the quantity, function, and membrane molecule TIM3 of the lamina propria dendritic cells (LPDCs). The regulatory effect of C. butyricum was verified in bone marrow-derived dendritic cells by in vitro experiments.
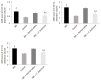
Results: The secretion of proinflammatory cytokines (IL-1β and IL-6) in mice with IBS was significantly increased compared with that of the control group, which suggested that the intestinal mucosa in mice with IBS was in a low-grade inflammatory state. The expression of CD11C+CD80+ and CD11c+TIM3+ in intestinal LPDCs in mice with IBS increased significantly. Meanwhile, the cytokines (IL-1β and IL-6) were significantly reduced after the intervention with probiotic C. butyricum. The amount and function of LPDCs and the TIM3 on the surface of the LPDCs were decreased with the alleviation of the intestinal inflammatory response.
Conclusion: The results suggest that C. butyricum regulates the amount and functional status of LPDCs in the intestinal mucosa of mice with IBS, and therefore modulates the local immune response in the intestine.
Keywords: Clostridium butyricum; Irritable bowel syndrome; Lamina propria dendritic cells; Proinflammatory cytokines; T cell immunoglobulin and mucin domain-3.
©The Author(s) 2019. Published by Baishideng Publishing Group Inc. All rights reserved.
Conflict of interest statement
Conflict-of-interest statement: The authors declare no conflicts of interest related to this manuscript or its publication.
Figures


Similar articles
-
Characteristics of intestinal lamina propria dendritic cells in a mouse model of postinfectious irritable bowel syndrome.J Gastroenterol Hepatol. 2012 May;27(5):935-44. doi: 10.1111/j.1440-1746.2011.07046.x. J Gastroenterol Hepatol. 2012. PMID: 22141367
-
Clostridium butyricum regulates visceral hypersensitivity of irritable bowel syndrome by inhibiting colonic mucous low grade inflammation through its action on NLRP6.Acta Biochim Biophys Sin (Shanghai). 2018 Feb 1;50(2):216-223. doi: 10.1093/abbs/gmx138. Acta Biochim Biophys Sin (Shanghai). 2018. PMID: 29329362
-
Corticotropin-releasing factor induces inflammatory cytokines via the NLRP6-inflammatory cytokine axis in a murine model of irritable bowel syndrome.J Dig Dis. 2019 Mar;20(3):143-151. doi: 10.1111/1751-2980.12704. Epub 2019 Mar 5. J Dig Dis. 2019. PMID: 30663229
-
Butyrate-producing human gut symbiont, Clostridium butyricum, and its role in health and disease.Gut Microbes. 2021 Jan-Dec;13(1):1-28. doi: 10.1080/19490976.2021.1907272. Gut Microbes. 2021. PMID: 33874858 Free PMC article. Review.
-
Probiotics and irritable bowel syndrome: rationale, mechanisms, and efficacy.J Clin Gastroenterol. 2008 Sep;42 Suppl 3 Pt 1:S123-5. doi: 10.1097/MCG.0b013e3181574393. J Clin Gastroenterol. 2008. PMID: 18806702 Review.
Cited by
-
Clostridium butyricum isolated from giant panda can attenuate dextran sodium sulfate-induced colitis in mice.Front Microbiol. 2024 Apr 5;15:1361945. doi: 10.3389/fmicb.2024.1361945. eCollection 2024. Front Microbiol. 2024. PMID: 38646621 Free PMC article.
-
Mesenchymal Stem Cell-derived Exosomes: Novel Therapeutic Approach for Inflammatory Bowel Diseases.Stem Cells Int. 2023 Apr 4;2023:4245704. doi: 10.1155/2023/4245704. eCollection 2023. Stem Cells Int. 2023. PMID: 37056457 Free PMC article. Review.
-
Clostridium butyricum Regulates the Inflammatory and Immunoregulatory Pathway Through NFKB1 in Colorectal Cancer Treatment.Probiotics Antimicrob Proteins. 2025 Apr 25. doi: 10.1007/s12602-025-10547-w. Online ahead of print. Probiotics Antimicrob Proteins. 2025. PMID: 40279041
-
The Many Ages of Microbiome-Gut-Brain Axis.Nutrients. 2022 Jul 18;14(14):2937. doi: 10.3390/nu14142937. Nutrients. 2022. PMID: 35889894 Free PMC article.
-
The Potential Role of Probiotics, Especially Butyrate Producers, in the Management of Gastrointestinal Mucositis Induced by Oncologic Chemo-Radiotherapy.Int J Mol Sci. 2024 Feb 15;25(4):2306. doi: 10.3390/ijms25042306. Int J Mol Sci. 2024. PMID: 38396981 Free PMC article.
References
-
- Sperber AD, Dumitrascu D, Fukudo S, Gerson C, Ghoshal UC, Gwee KA, Hungin APS, Kang JY, Minhu C, Schmulson M, Bolotin A, Friger M, Freud T, Whitehead W. The global prevalence of IBS in adults remains elusive due to the heterogeneity of studies: a Rome Foundation working team literature review. Gut. 2017;66:1075–1082. - PubMed
-
- Jeffery IB, O'Toole PW, Öhman L, Claesson MJ, Deane J, Quigley EM, Simrén M. An irritable bowel syndrome subtype defined by species-specific alterations in faecal microbiota. Gut. 2012;61:997–1006. - PubMed
-
- Vivinus-Nébot M, Frin-Mathy G, Bzioueche H, Dainese R, Bernard G, Anty R, Filippi J, Saint-Paul MC, Tulic MK, Verhasselt V, Hébuterne X, Piche T. Functional bowel symptoms in quiescent inflammatory bowel diseases: role of epithelial barrier disruption and low-grade inflammation. Gut. 2014;63:744–752. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

