Effects of shokyo (Zingiberis Rhizoma) and kankyo (Zingiberis Processum Rhizoma) on prostaglandin E2 production in lipopolysaccharide-treated mouse macrophage RAW264.7 cells
- PMID: 31576251
- PMCID: PMC6753926
- DOI: 10.7717/peerj.7725
Effects of shokyo (Zingiberis Rhizoma) and kankyo (Zingiberis Processum Rhizoma) on prostaglandin E2 production in lipopolysaccharide-treated mouse macrophage RAW264.7 cells
Abstract
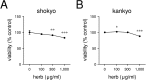
We previously reported that shokyo and kankyo, which are water-extracted fractions of ginger, reduced LPS-induced PGE2 production in human gingival fibroblasts. In this study, we examined the effects of these herbs on LPS-treated mouse macrophage RAW264.7 cells. Both shokyo and kankyo reduced LPS-induced PGE2 production in a concentration-dependent manner. Shokyo and kankyo did not inhibit cyclooxygenase (COX) activity, nor did they alter the expression of molecules in the arachidonic acid cascade. In addition, these herbs did not alter NF-κB p65 translocation into nucleus, or phosphorylation of p65 or ERK. These results suggest that shokyo and kankyo inhibit cPLA2 activity. Although 6-shogaol produced similar results to those of shokyo and kankyo, the concentration of 6-shogaol required for the reduction of PGE2 production were higher than those of 6-shogaol in shokyo and kankyo. Therefore, several gingerols and shogaols other than 6-shogaol may play a role in the reduction of LPS-induced PGE2 production. Thus, 6-shogaol, and other gingerols and shogaols inhibit cPLA2 activity and reduce LPS-induced PGE2 production via a different mechanism from traditional anti-inflammatory drugs. Moreover, kampo medicines that contain shokyo or kankyo are considered to be effective for inflammatory diseases.
Keywords: 6-shogaol; Antiinflammatory effect; Arachidonic acid cascade; Herb; Kampo medicine; Kankyo; Macrophage; Prostaglandin E2; Shokyo.
©2019 Ara et al.
Conflict of interest statement
The authors declare there are no competing interests.
Figures

Similar articles
-
Effects of shinbuto and ninjinto on prostaglandin E2 production in lipopolysaccharide-treated human gingival fibroblasts.PeerJ. 2017 Dec 1;5:e4120. doi: 10.7717/peerj.4120. eCollection 2017. PeerJ. 2017. PMID: 29209578 Free PMC article.
-
Studies on Shokyo, Kanzo, and Keihi in Kakkonto Medicine on Prostaglandin E2 Production in Lipopolysaccharide-Treated Human Gingival Fibroblasts.Int Sch Res Notices. 2016 Oct 13;2016:9351787. doi: 10.1155/2016/9351787. eCollection 2016. Int Sch Res Notices. 2016. PMID: 27819025 Free PMC article.
-
The Biological Efficacy of Natural Products against Acute and Chronic Inflammatory Diseases in the Oral Region.Medicines (Basel). 2018 Nov 13;5(4):122. doi: 10.3390/medicines5040122. Medicines (Basel). 2018. PMID: 30428613 Free PMC article. Review.
-
Pharmacological evaluation of Shokyo and Kankyo (1).Biol Pharm Bull. 2002 Sep;25(9):1183-7. doi: 10.1248/bpb.25.1183. Biol Pharm Bull. 2002. PMID: 12230114
-
Preventive Effects of a Kampo Medicine, Kakkonto, on Inflammatory Responses via the Suppression of Extracellular Signal-Regulated Kinase Phosphorylation in Lipopolysaccharide-Treated Human Gingival Fibroblasts.ISRN Pharmacol. 2014 Feb 18;2014:784019. doi: 10.1155/2014/784019. eCollection 2014. ISRN Pharmacol. 2014. PMID: 24693448 Free PMC article.
References
-
- Afzal M, Al-Hadidi D, Menon M, Pesek J, Dhami M. Ginger: an ethnomedical, chemical and pharmacological review. Drug Metabolism and Drug Interactions. 2001;18(3–4):159–190. - PubMed
-
- Aimbire F, Penna S, Rodrigues M, Rodrigues K, Lopes-Martins R, Sertié J. Effect of hydroalcoholic extract of Zingiber officinalis rhizomes on LPS-induced rat airway hyperreactivity and lung inflammation. Prostaglandins Leukot Essent Fatty Acids. 2007;77(3–4):129–138. doi: 10.1016/j.plefa.2007.08.008. - DOI - PubMed
-
- Alsherbiny M, Abd-Elsalam W, El Badawy S, Taher E, Fares M, Torres A, Chang D, Li C. Ameliorative and protective effects of ginger and its main constituents against natural, chemical and radiation-induced toxicities: a comprehensive review. Food and Chemical Toxicology. 2019;123:72–97. doi: 10.1016/j.fct.2018.10.048. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

