Cryo-electron microscopy reveals informative details of GABAA receptor structural pharmacology: implications for drug discovery
- PMID: 31576351
- PMCID: PMC6685854
- DOI: 10.21037/atm.2019.06.23
Cryo-electron microscopy reveals informative details of GABAA receptor structural pharmacology: implications for drug discovery
Conflict of interest statement
Conflicts of Interest: The authors have no conflicts of interest to declare.
Figures
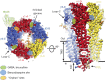
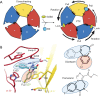
Comment on
-
GABAA receptor signalling mechanisms revealed by structural pharmacology.Nature. 2019 Jan;565(7740):454-459. doi: 10.1038/s41586-018-0832-5. Epub 2019 Jan 2. Nature. 2019. PMID: 30602790 Free PMC article.
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
