Nitrate and Phosphate Transporters Rescue Fluoride Toxicity in Yeast
- PMID: 31576749
- PMCID: PMC7316403
- DOI: 10.1021/acs.chemrestox.9b00315
Nitrate and Phosphate Transporters Rescue Fluoride Toxicity in Yeast
Abstract
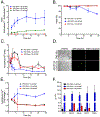
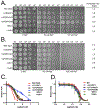
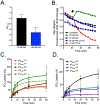
Organisms are exposed to fluoride in the air, water, and soil. Yeast and other microbes utilize fluoride channels as a method to prevent intracellular fluoride accumulation and mediate fluoride toxicity. Consequently, deletion of fluoride exporter genes (FEX) in S. cerevisiae resulted in over 1000-fold increased fluoride sensitivity. We used this FEX knockout strain to identify genes, that when overexpressed, are able to partially relieve the toxicity of fluoride exposure. Overexpression of five genes, SSU1, YHB1, IPP1, PHO87, and PHO90, increase fluoride tolerance by 2- to 10-fold. Overexpression of these genes did not provide improved fluoride resistance in wild-type yeast, suggesting that the mechanism is specific to low fluoride toxicity in yeast. Ssu1p and Yhb1p both function in nitrosative stress response, which is induced upon fluoride exposure along with metal influx. Ipp1p, Pho87p, and Pho90p increase intracellular orthophosphate. Consistent with this observation, fluoride toxicity is also partially mitigated by the addition of high levels of phosphate to the growth media. Fluoride inhibits phosphate import upon stress induction and causes nutrient starvation and organelle disruption, as supported by gene induction monitored through RNA-Seq. The combination of observations suggests that transmembrane nutrient transporters are among the most sensitized proteins during fluoride-instigated stress.
Figures




Similar articles
-
Flavohemoglobin expression and function in Saccharomyces cerevisiae. No relationship with respiration and complex response to oxidative stress.J Biol Chem. 1998 Apr 17;273(16):9527-33. doi: 10.1074/jbc.273.16.9527. J Biol Chem. 1998. PMID: 9545281
-
Function and expression of flavohemoglobin in Saccharomyces cerevisiae. Evidence for a role in the oxidative stress response.J Biol Chem. 1996 Oct 11;271(41):25131-8. doi: 10.1074/jbc.271.41.25131. J Biol Chem. 1996. PMID: 8810268
-
Conditional regulation of Puf1p, Puf4p, and Puf5p activity alters YHB1 mRNA stability for a rapid response to toxic nitric oxide stress in yeast.Mol Biol Cell. 2015 Mar 15;26(6):1015-29. doi: 10.1091/mbc.E14-10-1452. Epub 2015 Jan 28. Mol Biol Cell. 2015. PMID: 25631823 Free PMC article.
-
Inorganic Phosphate and Sulfate Transport in S. cerevisiae.Adv Exp Med Biol. 2016;892:253-269. doi: 10.1007/978-3-319-25304-6_10. Adv Exp Med Biol. 2016. PMID: 26721277 Review.
-
Phosphate homeostasis in the yeast Saccharomyces cerevisiae, the key role of the SPX domain-containing proteins.FEBS Lett. 2012 Feb 17;586(4):289-95. doi: 10.1016/j.febslet.2012.01.036. Epub 2012 Jan 27. FEBS Lett. 2012. PMID: 22285489 Review.
Cited by
-
Principles of fluoride toxicity and the cellular response: a review.Arch Toxicol. 2020 Apr;94(4):1051-1069. doi: 10.1007/s00204-020-02687-5. Epub 2020 Mar 9. Arch Toxicol. 2020. PMID: 32152649 Free PMC article. Review.
-
Tomato seed bio-priming with Pseudomonas aeruginosa strain PAR: a study on plant growth parameters under sodium fluoride stress.Front Microbiol. 2024 Jan 4;14:1330071. doi: 10.3389/fmicb.2023.1330071. eCollection 2023. Front Microbiol. 2024. PMID: 38239735 Free PMC article.
-
Engineering Inorganic Pyrophosphate Metabolism as a Strategy to Generate a Fluoride-Resistant Saccharomyces cerevisiae Strain.Microorganisms. 2025 Jan 21;13(2):226. doi: 10.3390/microorganisms13020226. Microorganisms. 2025. PMID: 40005593 Free PMC article.
-
Role of the CrcB transporter of Pseudomonas putida in the multi-level stress response elicited by mineral fluoride.Environ Microbiol. 2022 Nov;24(11):5082-5104. doi: 10.1111/1462-2920.16110. Epub 2022 Jul 1. Environ Microbiol. 2022. PMID: 35726888 Free PMC article.
-
The link between ancient microbial fluoride resistance mechanisms and bioengineering organofluorine degradation or synthesis.Nat Commun. 2024 May 30;15(1):4593. doi: 10.1038/s41467-024-49018-1. Nat Commun. 2024. PMID: 38816380 Free PMC article. Review.
References
-
- Smith FA, and Hodge HC (1979) Airborne fluorides and man. CRC Crit. Rev. Envir. Cont 8, 293–271.
-
- Jha SK, Mishra VK, Sharma DK, and Damodaran T (2011) Fluoride in the environment and its metabolism in humans. Rev. Environ. Contam. Toxicol 211, 121–142. - PubMed
-
- Vithanage M, and Bhattacharya P (2015) Fluoride in the environment: sources, distribution and defluoridation. Environ. Chem. Letters 13, 131–147.
-
- U.S. Department of Health and Human Services Federal Panel on Community Water Fluoridation (2015). U.S. Public Health Service recommendation for fluoride concentration in drinking water for the prevention of dental caries. Public health reports (Washington, D.C.: 1974), 130(4), 318–331. DOI: 10.1177/003335491513000408. - DOI - PMC - PubMed
-
- Centers for Disease Control and Prevention. (1999) Ten great public health achievements: United States, 1900–1999. Morbidity and Mortality Weekly Report 48, 241–243. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

